Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2 Theoretical background<br />
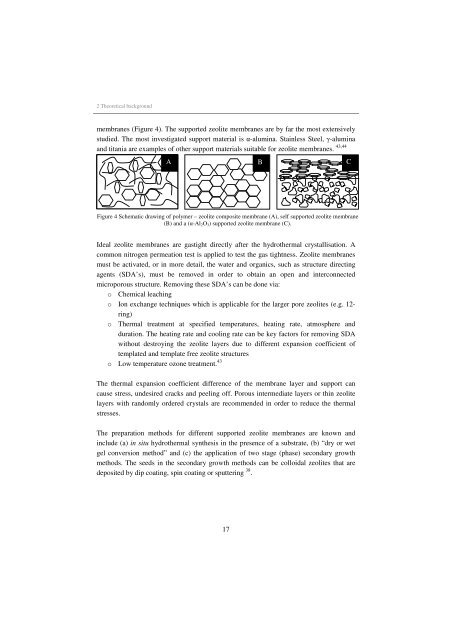
membranes (Figure 4). The supported zeolite membranes are by far the most extensively<br />
studied. The most <strong>in</strong>vestigated support material is α-alum<strong>in</strong>a. Sta<strong>in</strong>less Steel, γ-alum<strong>in</strong>a<br />
and titania are examples of other support materials suitable <strong>for</strong> zeolite membranes. 43,44<br />
A B C<br />
Figure 4 Schematic draw<strong>in</strong>g of polymer – zeolite composite membrane (A), self supported zeolite membrane<br />
(B) and a (α-Al2O3) supported zeolite membrane (C).<br />
Ideal zeolite membranes are gastight directly after the hydrothermal crystallisation. A<br />
common nitrogen permeation test is applied to test the gas tightness. Zeolite membranes<br />
must be activated, or <strong>in</strong> more detail, the water and organics, such as structure direct<strong>in</strong>g<br />
agents (SDA’s), must be removed <strong>in</strong> order to obta<strong>in</strong> an open and <strong>in</strong>terconnected<br />
microporous structure. Remov<strong>in</strong>g these SDA’s can be done via:<br />
o Chemical leach<strong>in</strong>g<br />
o Ion exchange techniques which is applicable <strong>for</strong> the larger pore zeolites (e.g. 12r<strong>in</strong>g)<br />
o Thermal treatment at specified temperatures, heat<strong>in</strong>g rate, atmosphere and<br />
duration. The heat<strong>in</strong>g rate and cool<strong>in</strong>g rate can be key factors <strong>for</strong> remov<strong>in</strong>g SDA<br />
without destroy<strong>in</strong>g the zeolite layers due to different expansion coefficient of<br />
templated and template free zeolite structures<br />
o Low temperature ozone treatment. 43<br />
The thermal expansion coefficient difference of the membrane layer and support can<br />
cause stress, undesired cracks and peel<strong>in</strong>g off. Porous <strong>in</strong>termediate layers or th<strong>in</strong> zeolite<br />
layers with randomly ordered crystals are recommended <strong>in</strong> order to reduce the thermal<br />
stresses.<br />
The preparation methods <strong>for</strong> different supported zeolite membranes are known and<br />
<strong>in</strong>clude (a) <strong>in</strong> situ hydrothermal synthesis <strong>in</strong> the presence of a substrate, (b) “dry or wet<br />
gel conversion method” and (c) the application of two stage (phase) secondary growth<br />
methods. The seeds <strong>in</strong> the secondary growth methods can be colloidal zeolites that are<br />
deposited by dip coat<strong>in</strong>g, sp<strong>in</strong> coat<strong>in</strong>g or sputter<strong>in</strong>g 38 .<br />
17