Gene regulation in Streptococcus pneumoniae - RePub - Erasmus ...
Gene regulation in Streptococcus pneumoniae - RePub - Erasmus ...
Gene regulation in Streptococcus pneumoniae - RePub - Erasmus ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 6<br />
homologue is present, <strong>in</strong>dicat<strong>in</strong>g differences <strong>in</strong> <strong>regulation</strong> of nitrogen metabolism with B.<br />
subtilis.<br />
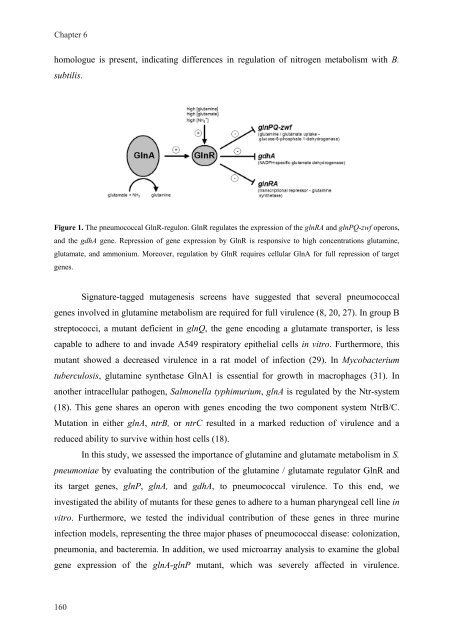
Figure 1. The pneumococcal GlnR-regulon. GlnR regulates the expression of the glnRA and glnPQ-zwf operons,<br />
and the gdhA gene. Repression of gene expression by GlnR is responsive to high concentrations glutam<strong>in</strong>e,<br />
glutamate, and ammonium. Moreover, <strong>regulation</strong> by GlnR requires cellular GlnA for full repression of target<br />
genes.<br />
Signature-tagged mutagenesis screens have suggested that several pneumococcal<br />
genes <strong>in</strong>volved <strong>in</strong> glutam<strong>in</strong>e metabolism are required for full virulence (8, 20, 27). In group B<br />
streptococci, a mutant deficient <strong>in</strong> glnQ, the gene encod<strong>in</strong>g a glutamate transporter, is less<br />
capable to adhere to and <strong>in</strong>vade A549 respiratory epithelial cells <strong>in</strong> vitro. Furthermore, this<br />
mutant showed a decreased virulence <strong>in</strong> a rat model of <strong>in</strong>fection (29). In Mycobacterium<br />
tuberculosis, glutam<strong>in</strong>e synthetase GlnA1 is essential for growth <strong>in</strong> macrophages (31). In<br />
another <strong>in</strong>tracellular pathogen, Salmonella typhimurium, glnA is regulated by the Ntr-system<br />
(18). This gene shares an operon with genes encod<strong>in</strong>g the two component system NtrB/C.<br />
Mutation <strong>in</strong> either glnA, ntrB, or ntrC resulted <strong>in</strong> a marked reduction of virulence and a<br />
reduced ability to survive with<strong>in</strong> host cells (18).<br />
In this study, we assessed the importance of glutam<strong>in</strong>e and glutamate metabolism <strong>in</strong> S.<br />
<strong>pneumoniae</strong> by evaluat<strong>in</strong>g the contribution of the glutam<strong>in</strong>e / glutamate regulator GlnR and<br />
its target genes, glnP, glnA, and gdhA, to pneumococcal virulence. To this end, we<br />
<strong>in</strong>vestigated the ability of mutants for these genes to adhere to a human pharyngeal cell l<strong>in</strong>e <strong>in</strong><br />
vitro. Furthermore, we tested the <strong>in</strong>dividual contribution of these genes <strong>in</strong> three mur<strong>in</strong>e<br />
<strong>in</strong>fection models, represent<strong>in</strong>g the three major phases of pneumococcal disease: colonization,<br />
pneumonia, and bacteremia. In addition, we used microarray analysis to exam<strong>in</strong>e the global<br />
gene expression of the glnA-glnP mutant, which was severely affected <strong>in</strong> virulence.<br />
160<br />
160