International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Metathesis in Water – A Case Study for<br />
Supramolecular Modification of Reactivity<br />
Miriam Sessler, Jürgen Schatz<br />
Organic Chemistry 1, Department Chemistry and Pharmacy,<br />
University of Erlangen, Henkestr. 42, 91054 Erlangen / Germany<br />
Generally, metathesis reactions are carried out in organic solvents and their potential<br />
utilization in aqueous medium is largely unexploited. The majority of methods reported<br />
for aqueous metathesis did not use pure water as a reaction medium but only organic<br />
solvents with some percentage of water in it. [1] However, the idea of “green chemistry”<br />
has encouraged us to develop a simple and environmentally benign ring-closing<br />
metathesis (RCM) protocol based exclusively on aqueous media. [2]<br />
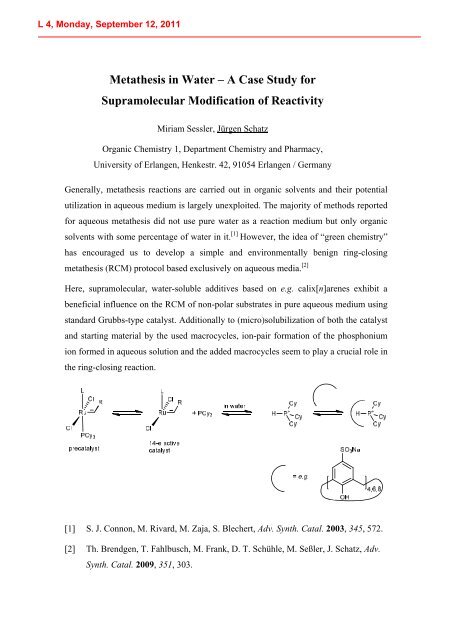
Here, supramolecular, water-soluble additives based on e.g. calix[n]arenes exhibit a<br />
beneficial influence on the RCM of non-polar substrates in pure aqueous medium using<br />
standard Grubbs-type catalyst. Additionally to (micro)solubilization of both the catalyst<br />
and starting material by the used macrocycles, ion-pair formation of the phosphonium<br />
ion formed in aqueous solution and the added macrocycles seem to play a crucial role in<br />
the ring-closing reaction.<br />
[1] S. J. Connon, M. Rivard, M. Zaja, S. Blechert, Adv. Synth. Catal. 2003, 345, 572.<br />
[2] Th. Brendgen, T. Fahlbusch, M. Frank, D. T. Schühle, M. Seßler, J. Schatz, Adv.<br />
Synth. Catal. 2009, 351, 303.