International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1<br />
Synthesis and Cation-Dependent Photophysical Properties of<br />
Crown-Containing 1,8-Naphthalimide Derivatives<br />
P. A. Panchenko 1 *, Yu. V. Fedorov 1 , O. A. Fedorova 1 , G. Jonusauskas 2<br />
Institute of Organoelement Compounds of Russian Academy of Sciences, Vavilova 28,<br />
Moscow, 119991, Russia. E-mail: pavel@ineos.ac.ru<br />
2<br />
Centre de Physique Moléculaire Optique et Hertzienne (CPMOH), UMR CNRS 5798,<br />
Université Bordeaux 1, 351, Cours de la Libération, 33405 Talence, France<br />
During the last years, much attention has been paid to the design, synthesis and<br />
characterization of photochemical molecular devices, which combine chromophore<br />
signaling units and receptor groups capable of binding of molecules and ions. Such<br />
devices can find employment in many disciplines such as biochemistry, clinical and<br />
medical sciences, analytical chemistry and environmental science.<br />
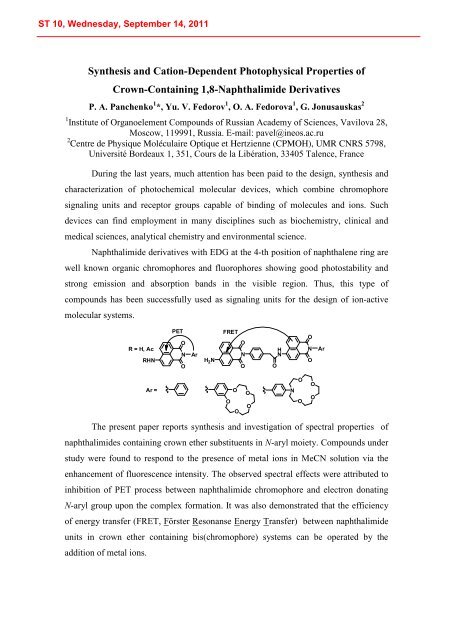
Naphthalimide derivatives with EDG at the 4-th position of naphthalene ring are<br />
well known organic chromophores and fluorophores showing good photostability and<br />
strong emission and absorption bands in the visible region. Thus, this type of<br />
compounds has been successfully used as signaling units for the design of ion-active<br />
molecular systems.<br />
R = H, Ac<br />
RHN<br />
Ar =<br />
PET FRET<br />
O<br />
O<br />
N Ar<br />
O<br />
H 2 N<br />
O<br />
O<br />
N<br />
O<br />
O<br />
O O<br />
O<br />
H<br />
N<br />
O<br />
N<br />
O<br />
O<br />
O<br />
N<br />
O O<br />
The present paper reports synthesis and investigation of spectral properties of<br />
naphthalimides containing crown ether substituents in N-aryl moiety. Compounds under<br />
study were found to respond to the presence of metal ions in MeCN solution via the<br />
enhancement of fluorescence intensity. The observed spectral effects were attributed to<br />
inhibition of PET process between naphthalimide chromophore and electron donating<br />
N-aryl group upon the complex formation. It was also demonstrated that the efficiency<br />
of energy transfer (FRET, Förster Resonanse Energy Transfer) between naphthalimide<br />
units in crown ether containing bis(chromophore) systems can be operated by the<br />
addition of metal ions.<br />
Ar