International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Synthesis and investigation of complexing, optical and electrochemical activities of<br />
crown-containing oligothiophene derivatives<br />
Artemiy Mizerev a , Elena Lukovskaya a , Olga Fedorova a , Yury Fedorov b , Alla Bobylyova a , Anna<br />
Moiseeva a , Aleksander Anisimov a<br />
a<br />
Department of Chemistry, M.V. Lomonosov Moscow State University, Leninskie Gory, 119992 Moscow,<br />
Russia, E-mail: lukov@petrol.chem.msu.ru; Fax: +7 (495) 932 85 68<br />
b<br />
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, 28 Vavilova<br />
str., 119991, Moscow, Russia<br />
Functional oligothiophenes have attracted comprehensive interest among researchers all over the<br />
world and have actually been advanced to be among the most frequently used π-conjugated materials, in<br />
particular as active components in organic electronic devices and molecular electronics.<br />
Thiophene-containing donor-acceptor substituted systems show electron and energy transfer<br />
processes. The changes in physical properties (e.g., absorption, fuorescence, electrochemistry, etc.) of<br />
these derivatives strongly depended on the nature of both the π-conjugation and the type of donor-<br />
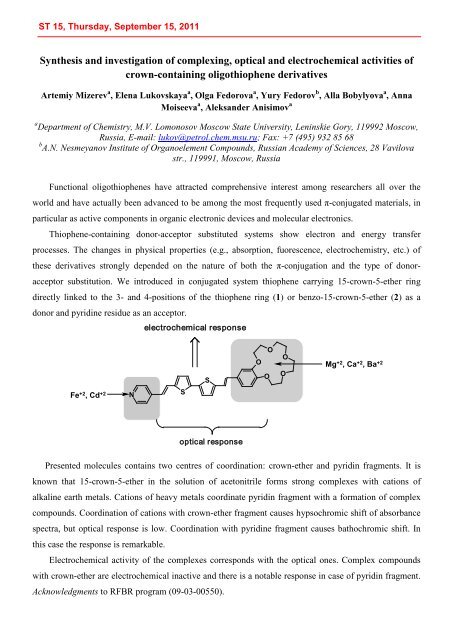
acceptor substitution. We introduced in conjugated system thiophene carrying 15-crown-5-ether ring<br />
directly linked to the 3- and 4-positions of the thiophene ring (1) or benzo-15-crown-5-ether (2) as a<br />
donor and pyridine residue as an acceptor.<br />
Fe +2 , Cd +2<br />
N<br />
electrochemical response<br />
S<br />
S<br />
optical response<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Mg +2 , Ca +2 , Ba +2<br />
Presented molecules contains two centres of coordination: crown-ether and pyridin fragments. It is<br />
known that 15-crown-5-ether in the solution of acetonitrile forms strong complexes with cations of<br />
alkaline earth metals. Cations of heavy metals coordinate pyridin fragment with a formation of complex<br />
compounds. Coordination of cations with crown-ether fragment causes hypsochromic shift of absorbance<br />
spectra, but optical response is low. Coordination with pyridine fragment causes bathochromic shift. In<br />
this case the response is remarkable.<br />
Electrochemical activity of the complexes corresponds with the optical ones. Complex compounds<br />
with crown-ether are electrochemical inactive and there is a notable response in case of pyridin fragment.<br />
Acknowledgments to RFBR program (09-03-00550).