International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Aromaticity Switching In Porphyrinoids And Heterorphyrinoids<br />
Lechosław Latos-Grażyński<br />
University of Wrocław, Wrocław, POLAND, llg@wchuwr.pl<br />
One of the ways to study the phenomenon of aromaticity is through the synthesis of new<br />
molecules, which are often specifically designed to test various aspects of the theory or<br />
to pose new problems. 1 Aromaticity of porphyrinoids can be influenced by a variety of<br />
structural modifications, such<br />
as peripheral substitution,<br />
covalent linking of multiple<br />
macrocycles, ring expansion<br />
or contraction, and<br />
introduction of non pyrrolic<br />
subunits. The latter approach<br />
is most readily realized by<br />
replacing one of the pyrrole<br />
rings of the porphyrin with a<br />
different hetero- or carbocyclic fragment. Such a modification can have a profound<br />
influence on the aromaticity of the macrocycle, as demonstrated by the investigated<br />
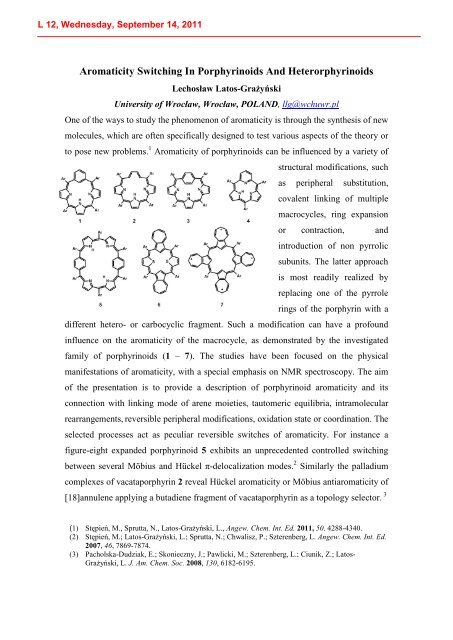
family of porphyrinoids (1 – 7). The studies have been focused on the physical<br />
manifestations of aromaticity, with a special emphasis on NMR spectroscopy. The aim<br />
of the presentation is to provide a description of porphyrinoid aromaticity and its<br />
connection with linking mode of arene moieties, tautomeric equilibria, intramolecular<br />
rearrangements, reversible peripheral modifications, oxidation state or coordination. The<br />
selected processes act as peculiar reversible switches of aromaticity. For instance a<br />
figure-eight expanded porphyrinoid 5 exhibits an unprecedented controlled switching<br />
between several Möbius and Hückel π-delocalization modes. 2 Similarly the palladium<br />
complexes of vacataporphyrin 2 reveal Hückel aromaticity or Möbius antiaromaticity of<br />
[18]annulene applying a butadiene fragment of vacataporphyrin as a topology selector. 3<br />
(1) Stępień, M., Sprutta, N., Latos-Grażyński, L., Angew. Chem. Int. Ed. 2011, 50, 4288-4340.<br />
(2) Stępień, M.; Latos-Grażyński, L.; Sprutta, N.; Chwalisz, P.; Szterenberg, L. Angew. Chem. Int. Ed.<br />
2007, 46, 7869-7874.<br />
(3) Pacholska-Dudziak, E.; Skonieczny, J.; Pawlicki, M.; Szterenberg, L.; Ciunik, Z.; Latos-<br />
Grażyński, L. J. Am. Chem. Soc. 2008, 130, 6182-6195.