International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Crown-containing derivatives of naphthopyrans: cation<br />
binding, photochemical and fluorescent properties<br />
A.B. Smolentsev 1 , E.M. Glebov 1 , V.V. Korolev 1 , S.V. Paramonov 2 , O.A. Fedorova 2<br />
1 – Institute of Chemichal Kinetics and Kombustion SB RAS, Novosibirsk , Russia<br />
2 - Nesmeyanov Institute of Organoelement Compounds RAS, Moscow, Russia<br />
S_art@ngs.ru<br />
Photochromic molecules containing macrocyclic moiety have attracted much<br />
interest in molecular chemistry as components of functional materials. Examples of<br />
potential applications of these compounds include: photoswitching transport through<br />
membranes, optical information storage, photoswitching extraction of metal cations.<br />
A<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
B<br />
O<br />
O N<br />
O O<br />
O<br />
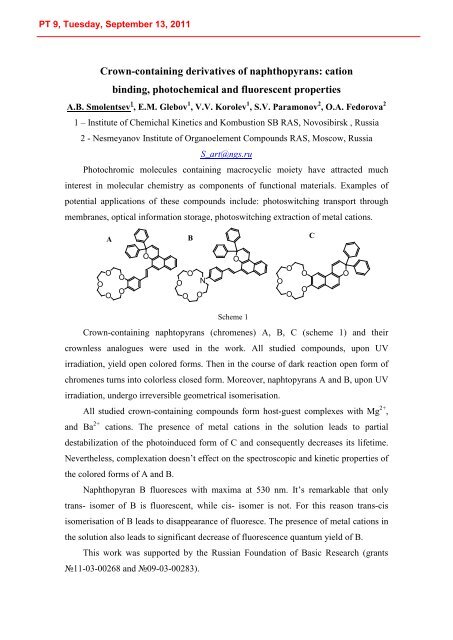
Scheme 1<br />
O<br />
O<br />
O<br />
O O<br />
Crown-containing naphtopyrans (chromenes) A, B, C (scheme 1) and their<br />
crownless analogues were used in the work. All studied compounds, upon UV<br />
irradiation, yield open colored forms. Then in the course of dark reaction open form of<br />
chromenes turns into colorless closed form. Moreover, naphtopyrans A and B, upon UV<br />
irradiation, undergo irreversible geometrical isomerisation.<br />
All studied crown-containing compounds form host-guest complexes with Mg 2+ ,<br />
and Ba 2+<br />
cations. The presence of metal cations in the solution leads to partial<br />
destabilization of the photoinduced form of С and consequently decreases its lifetime.<br />
Nevertheless, complexation doesn’t effect on the spectroscopic and kinetic properties of<br />
the colored forms of A and B.<br />
Naphthopyran B fluoresces with maxima at 530 nm. It’s remarkable that only<br />
trans- isomer of B is fluorescent, while cis- isomer is not. For this reason trans-cis<br />
isomerisation of B leads to disappearance of fluoresce. The presence of metal cations in<br />
the solution also leads to significant decrease of fluorescence quantum yield of B.<br />
This work was supported by the Russian Foundation of Basic Research (grants<br />
№11-03-00268 and №09-03-00283).<br />
C<br />
O