International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Synthesis and Investigations of Multiple Hydrogen Bonded<br />
Supramolecular Host - Guest Complexes<br />
Philipp Otte,<br />
Ulrich Lüning*<br />
Otto-Diels-Institut für Organische Chemie, Olshausenstraße 40, 24098 Kiel, GER.<br />
potte@oc.uni-kiel.de, luening@oc.uni-kiel.de<br />
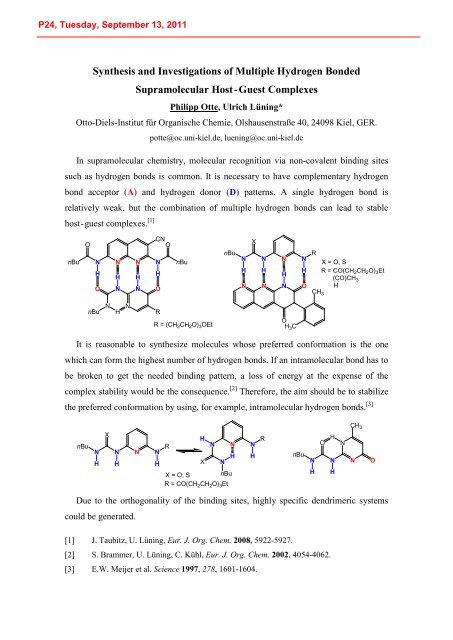
In supramolecular chemistry, molecular recognition via non-covalent binding sites<br />
such as hydrogen bonds is common. It is necessary to have complementary hydrogen<br />
bond acceptor (A) and hydrogen donor (D) patterns. A single hydrogen bond is<br />
relatively weak, but the combination of multiple hydrogen bonds can lead to stable<br />
host - guest complexes. [1]<br />
nBu<br />
O<br />
N<br />
H<br />
O<br />
nBu<br />
N N<br />
H H<br />
N N<br />
N H N<br />
CN<br />
O<br />
N<br />
H<br />
O<br />
R<br />
nBu<br />
R = (CH 2CH 2O) 3OEt<br />
nBu<br />
N<br />
H<br />
X<br />
N N N R<br />
H<br />
H<br />
H<br />
N N N O<br />
O<br />
H3C CH 3<br />
X = O, S<br />
R = CO(CH2CH2O) 3Et<br />
(CO)CH3 H<br />
It is reasonable to synthesize molecules whose preferred conformation is the one<br />
which can form the highest number of hydrogen bonds. If an intramolecular bond has to<br />
be broken to get the needed binding pattern, a loss of energy at the expense of the<br />
complex stability would be the consequence. [2] Therefore, the aim should be to stabilize<br />
[3]<br />
the preferred conformation by using, for example, intramolecular hydrogen bonds.<br />
nBu<br />
N<br />
H<br />
X<br />
N N N R<br />
H<br />
H<br />
H<br />
X<br />
N<br />
N<br />
X = O, S nBu<br />
R = CO(CH2CH2O) 3Et<br />
N N R<br />
H<br />
Due to the orthogonality of the binding sites, highly specific dendrimeric systems<br />
could be generated.<br />
[1] J. Taubitz, U. Lüning, Eur. J. Org. Chem. 2008, 5922-5927.<br />
[2] S. Brammer, U. Lüning, C. Kühl, Eur. J. Org. Chem. 2002, 4054-4062.<br />
[3] E.W. Meijer et al. Science 1997, 278, 1601-1604.<br />
H<br />
nBu<br />
N<br />
H<br />
O<br />
H<br />
N<br />
H<br />
N<br />
CH 3<br />
N<br />
O