International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
International Summer School PROGRAM - Laboratoire d'Infochimie ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Core-Modified Porphyrinoid: Syntheses and Characterization of<br />
Disilahexaphyrinoid<br />
Janusz Skonieczny,<br />
Lechosław Latos-Grażyński, Ludmiła Szterenberg<br />
Department of Chemistry<br />
University of Wrocław<br />
14 F. Joliot-Curie St., Wrocław 50 383, Poland<br />
e-mail: LLG@wchuwr.pl<br />
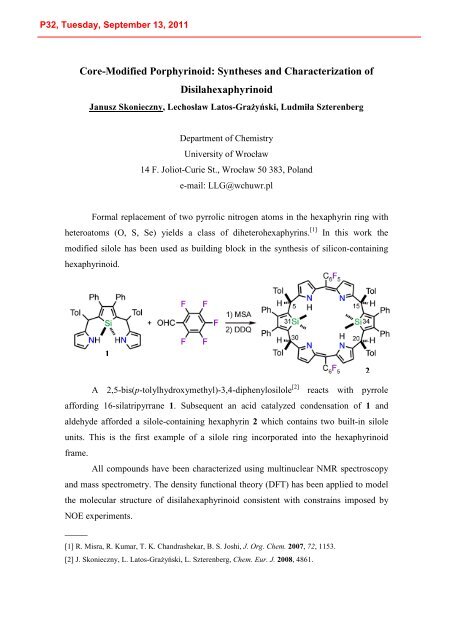
Formal replacement of two pyrrolic nitrogen atoms in the hexaphyrin ring with<br />
heteroatoms (O, S, Se) yields a class of diheterohexaphyrins. [1]<br />
In this work the<br />
modified silole has been used as building block in the synthesis of silicon-containing<br />
hexaphyrinoid.<br />
1<br />
A 2,5-bis(p-tolylhydroxymethyl)-3,4-diphenylosilole [2]<br />
reacts with pyrrole<br />
affording 16-silatripyrrane 1. Subsequent an acid catalyzed condensation of 1 and<br />
aldehyde afforded a silole-containing hexaphyrin 2 which contains two built-in silole<br />
units. This is the first example of a silole ring incorporated into the hexaphyrinoid<br />
frame.<br />
All compounds have been characterized using multinuclear NMR spectroscopy<br />
and mass spectrometry. The density functional theory (DFT) has been applied to model<br />
the molecular structure of disilahexaphyrinoid consistent with constrains imposed by<br />
NOE experiments.<br />
_____<br />
[1] R. Misra, R. Kumar, T. K. Chandrashekar, B. S. Joshi, J. Org. Chem. 2007, 72, 1153.<br />
[2] J. Skonieczny, L. Latos-Grażyński, L. Szterenberg, Chem. Eur. J. 2008, 4861.<br />
2