© 2006 by Taylor & Francis Group, LLC
© 2006 by Taylor & Francis Group, LLC
© 2006 by Taylor & Francis Group, LLC
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Abrasive Blasting and Heavy-Metal Contamination 91<br />
While it is arguable that iron could form temporary, weak, ionic complexes…so that<br />
when analyzed <strong>by</strong> the TCLP test the lead appears to have been stabilized, the Agency<br />
believes that this ‘‘stabilization” is temporary, based upon the nature of the complexing.<br />
In fact, a report prepared <strong>by</strong> the EPA on Iron Chemistry in Lead-Contaminated Materials<br />
(Feb. 22 1994), which specifically addressed this issue, found that iron-lead bonds are<br />
weak, adsorptive surface bonds, and therefore not likely to be permanent. Furthermore,<br />
as this iron-rich mixture is exposed to moisture and oxidative conditions over time,<br />
interstitial water would likely acidify, which could potentially reverse any temporary<br />
stabilization, as well as increase the leachability of the lead…. Therefore, the addition<br />
of iron dust or filings to…waste…does not appear to provide long-term treatment.<br />
5.3.2 STABILIZATION OF LEAD THROUGH PH ADJUSTMENT<br />
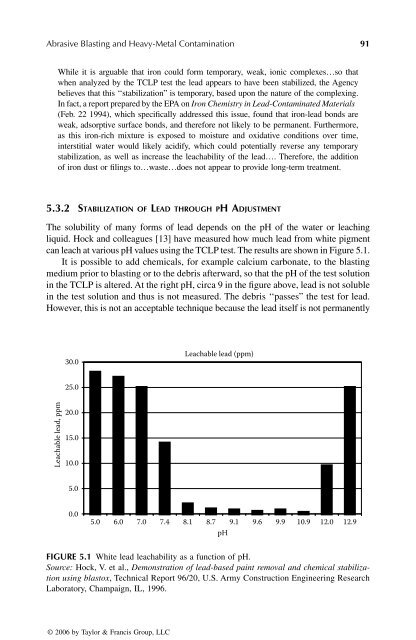
The solubility of many forms of lead depends on the pH of the water or leaching<br />
liquid. Hock and colleagues [13] have measured how much lead from white pigment<br />
can leach at various pH values using the TCLP test. The results are shown in Figure 5.1.<br />
It is possible to add chemicals, for example calcium carbonate, to the blasting<br />
medium prior to blasting or to the debris afterward, so that the pH of the test solution<br />
in the TCLP is altered. At the right pH, circa 9 in the figure above, lead is not soluble<br />
in the test solution and thus is not measured. The debris ‘‘passes” the test for lead.<br />
However, this is not an acceptable technique because the lead itself is not permanently<br />
Leachable lead, ppm<br />
30.0<br />
25.0<br />
20.0<br />
15.0<br />
10.0<br />
5.0<br />
0.0<br />
Leachable lead (ppm)<br />
5.0 6.0 7.0 7.4 8.1 8.7 9.1 9.6 9.9 10.9 12.0 12.9<br />
pH<br />
FIGURE 5.1 White lead leachability as a function of pH.<br />
Source: Hock, V. et al., Demonstration of lead-based paint removal and chemical stabilization<br />
using blastox, Technical Report 96/20, U.S. Army Construction Engineering Research<br />
Laboratory, Champaign, IL, 1996.<br />
<strong>©</strong> <strong>2006</strong> <strong>by</strong> <strong>Taylor</strong> & <strong>Francis</strong> <strong>Group</strong>, <strong>LLC</strong>