© 2006 by Taylor & Francis Group, LLC
© 2006 by Taylor & Francis Group, LLC
© 2006 by Taylor & Francis Group, LLC
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Corrosion Testing — Background and Theoretical Considerations 119<br />
ln (D)<br />
2<br />
1<br />
0<br />
0 20 40 60 80 100<br />
−1<br />
−2<br />
Rdry<br />
CRS, 0 Zn<br />
EGS, 20 Zn<br />
EGS, 40 Zn<br />
HDG, 90 Zn<br />
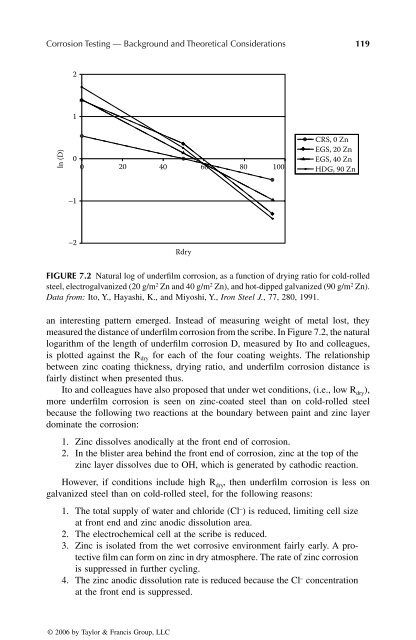
FIGURE 7.2 Natural log of underfilm corrosion, as a function of drying ratio for cold-rolled<br />
steel, electrogalvanized (20 g/m 2 Zn and 40 g/m 2 Zn), and hot-dipped galvanized (90 g/m 2 Zn).<br />
Data from: Ito, Y., Hayashi, K., and Miyoshi, Y., Iron Steel J., 77, 280, 1991.<br />
an interesting pattern emerged. Instead of measuring weight of metal lost, they<br />
measured the distance of underfilm corrosion from the scribe. In Figure 7.2, the natural<br />
logarithm of the length of underfilm corrosion D, measured <strong>by</strong> Ito and colleagues,<br />
is plotted against the R dry for each of the four coating weights. The relationship<br />
between zinc coating thickness, drying ratio, and underfilm corrosion distance is<br />
fairly distinct when presented thus.<br />
Ito and colleagues have also proposed that under wet conditions, (i.e., low R dry),<br />
more underfilm corrosion is seen on zinc-coated steel than on cold-rolled steel<br />
because the following two reactions at the boundary between paint and zinc layer<br />
dominate the corrosion:<br />
1. Zinc dissolves anodically at the front end of corrosion.<br />
2. In the blister area behind the front end of corrosion, zinc at the top of the<br />
zinc layer dissolves due to OH, which is generated <strong>by</strong> cathodic reaction.<br />
However, if conditions include high R dry, then underfilm corrosion is less on<br />
galvanized steel than on cold-rolled steel, for the following reasons:<br />
1. The total supply of water and chloride (Cl – ) is reduced, limiting cell size<br />
at front end and zinc anodic dissolution area.<br />
2. The electrochemical cell at the scribe is reduced.<br />
3. Zinc is isolated from the wet corrosive environment fairly early. A protective<br />
film can form on zinc in dry atmosphere. The rate of zinc corrosion<br />
is suppressed in further cycling.<br />
4. The zinc anodic dissolution rate is reduced because the Cl – concentration<br />
at the front end is suppressed.<br />
<strong>©</strong> <strong>2006</strong> <strong>by</strong> <strong>Taylor</strong> & <strong>Francis</strong> <strong>Group</strong>, <strong>LLC</strong>