© 2006 by Taylor & Francis Group, LLC
© 2006 by Taylor & Francis Group, LLC
© 2006 by Taylor & Francis Group, LLC
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
146 Corrosion Control Through Organic Coatings<br />
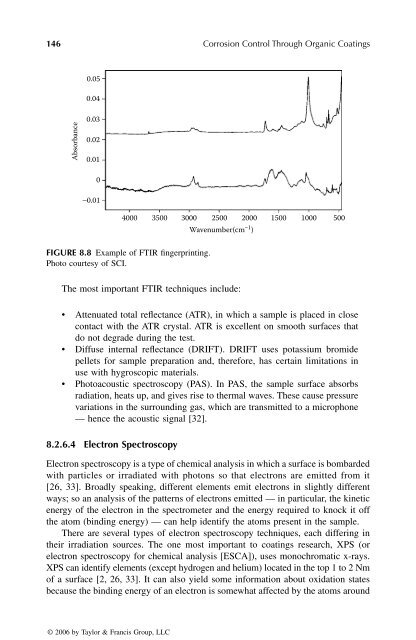
Absorbance<br />
0.05<br />
0.04<br />
0.03<br />
0.02<br />
0.01<br />
0<br />
−0.01<br />
FIGURE 8.8 Example of FTIR fingerprinting.<br />
Photo courtesy of SCI.<br />
The most important FTIR techniques include:<br />
• Attenuated total reflectance (ATR), in which a sample is placed in close<br />
contact with the ATR crystal. ATR is excellent on smooth surfaces that<br />
do not degrade during the test.<br />
• Diffuse internal reflectance (DRIFT). DRIFT uses potassium bromide<br />
pellets for sample preparation and, therefore, has certain limitations in<br />
use with hygroscopic materials.<br />
• Photoacoustic spectroscopy (PAS). In PAS, the sample surface absorbs<br />
radiation, heats up, and gives rise to thermal waves. These cause pressure<br />
variations in the surrounding gas, which are transmitted to a microphone<br />
— hence the acoustic signal [32].<br />
8.2.6.4 Electron Spectroscopy<br />
4000 3500 3000 2500 2000 1500 1000 500<br />
Wavenumber(cm−1 )<br />
Electron spectroscopy is a type of chemical analysis in which a surface is bombarded<br />
with particles or irradiated with photons so that electrons are emitted from it<br />
[26, 33]. Broadly speaking, different elements emit electrons in slightly different<br />
ways; so an analysis of the patterns of electrons emitted — in particular, the kinetic<br />
energy of the electron in the spectrometer and the energy required to knock it off<br />
the atom (binding energy) — can help identify the atoms present in the sample.<br />
There are several types of electron spectroscopy techniques, each differing in<br />
their irradiation sources. The one most important to coatings research, XPS (or<br />
electron spectroscopy for chemical analysis [ESCA]), uses monochromatic x-rays.<br />
XPS can identify elements (except hydrogen and helium) located in the top 1 to 2 Nm<br />
of a surface [2, 26, 33]. It can also yield some information about oxidation states<br />
because the binding energy of an electron is somewhat affected <strong>by</strong> the atoms around<br />
<strong>©</strong> <strong>2006</strong> <strong>by</strong> <strong>Taylor</strong> & <strong>Francis</strong> <strong>Group</strong>, <strong>LLC</strong>