Regulatory Requirements for Investigational Drugs - à¸à¸£à¸°à¸à¸£à¸§à¸à¸ªà¸²à¸à¸²à¸£à¸à¸ªà¸¸à¸
Regulatory Requirements for Investigational Drugs - à¸à¸£à¸°à¸à¸£à¸§à¸à¸ªà¸²à¸à¸²à¸£à¸à¸ªà¸¸à¸
Regulatory Requirements for Investigational Drugs - à¸à¸£à¸°à¸à¸£à¸§à¸à¸ªà¸²à¸à¸²à¸£à¸à¸ªà¸¸à¸
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Thailand<br />
Food &<br />
Drug<br />
Administration<br />
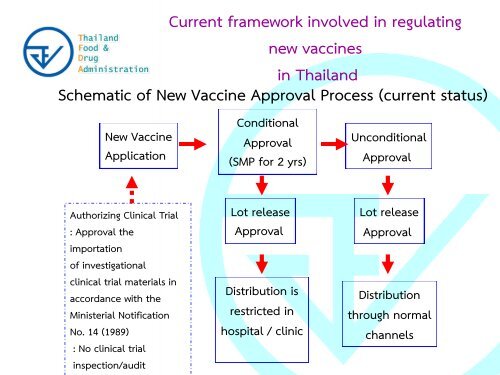
Current framework involved in regulating<br />
new vaccines<br />
in Thailand<br />
Schematic of New Vaccine Approval Process (current status)<br />
New Vaccine<br />
Application<br />
Conditional<br />
Approval<br />
(SMP <strong>for</strong> 2 yrs)<br />
Unconditional<br />
Approval<br />
Authorizing Clinical Trial<br />
: Approval the<br />
importation<br />
of investigational<br />
clinical trial materials in<br />
accordance with the<br />
Ministerial Notification<br />
No. 14 (1989)<br />
: No clinical trial<br />
inspection/audit<br />
Lot release<br />
Approval<br />
Distribution is<br />
restricted in<br />
hospital / clinic<br />
Lot release<br />
Approval<br />
Distribution<br />
through normal<br />
channels