Regulatory Requirements for Investigational Drugs - à¸à¸£à¸°à¸à¸£à¸§à¸à¸ªà¸²à¸à¸²à¸£à¸à¸ªà¸¸à¸
Regulatory Requirements for Investigational Drugs - à¸à¸£à¸°à¸à¸£à¸§à¸à¸ªà¸²à¸à¸²à¸£à¸à¸ªà¸¸à¸
Regulatory Requirements for Investigational Drugs - à¸à¸£à¸°à¸à¸£à¸§à¸à¸ªà¸²à¸à¸²à¸£à¸à¸ªà¸¸à¸
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
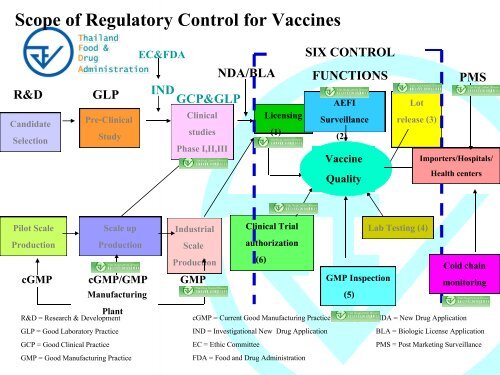
Scope of <strong>Regulatory</strong> Control <strong>for</strong> Vaccines<br />
R&D<br />
Candidate<br />
Selection<br />
Thailand<br />
Food &<br />
Drug<br />
Administration<br />
GLP<br />
Pre-Clinical<br />
Study<br />
EC&FDA<br />
NDA/BLA<br />
IND GCP&GLP<br />
Clinical<br />
studies<br />
Phase I,II,III<br />
Licensing<br />
(1)<br />
SIX CONTROL<br />
FUNCTIONS<br />
AEFI<br />
Surveillance<br />
(2)<br />
Vaccine<br />
Quality<br />
Lot<br />
release (3)<br />
PMS<br />
Importers/Hospitals/<br />
Health centers<br />
Pilot Scale<br />
Production<br />
Scale up<br />
Production<br />
Manufacturing<br />
Plant<br />
Industrial<br />
Scale<br />
Production<br />
cGMP cGMP/GMP GMP<br />
R&D = Research & Development<br />
GLP = Good Laboratory Practice<br />
GCP = Good Clinical Practice<br />
GMP = Good Manufacturing Practice<br />
Clinical Trial<br />
authorization<br />
(6)<br />
cGMP = Current Good Manufacturing Practice<br />
IND = <strong>Investigational</strong> New Drug Application<br />
EC = Ethic Committee<br />
FDA = Food and Drug Administration<br />
GMP Inspection<br />
(5)<br />
Lab Testing (4)<br />
Cold chain<br />
monitoring<br />
NDA = New Drug Application<br />
BLA = Biologic License Application<br />
PMS = Post Marketing Surveillance