Anthony KERMAGORET - THESES ET MEMOIRES DE L'UDS
Anthony KERMAGORET - THESES ET MEMOIRES DE L'UDS
Anthony KERMAGORET - THESES ET MEMOIRES DE L'UDS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapitre VI<br />
The synthesis of β-keto phosphorus ylids from mixed alkyl/phenyl phosphonium salts<br />
could provide an access to SHOP-type nickel complexes having alkyl or mixed alkyl/phenyl<br />
substituents on the P atom of the P,O chelate. Such mixed salts were prepared by reaction of<br />
PPh2Cl with one equiv. of n-BuLi or t-BuLi or by reaction of PPhCl2 with two equiv. of n-<br />
BuLi or t-BuLi at low temperature, 55 followed by reaction with α-bromoacetophenone and<br />
deprotonation. The β-keto phosphorus ylids 7 and 8, with t-butyl substituents, precipitated as<br />
white powders but 9 and 10, with n-butyl substituents, formed a pale red oil.<br />
We did not succeed in deprotonating methyl-substituted phosphonium salts using a<br />
MeOH/H2O (1/1) solution of NaOH but the β-keto phosphorus ylid 11 was obtained in<br />
toluene using NaH.<br />
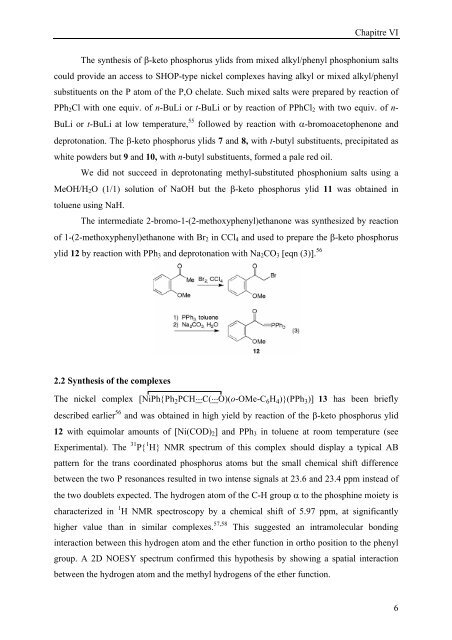
The intermediate 2-bromo-1-(2-methoxyphenyl)ethanone was synthesized by reaction<br />
of 1-(2-methoxyphenyl)ethanone with Br2 in CCl4 and used to prepare the β-keto phosphorus<br />
ylid 12 by reaction with PPh3 and deprotonation with Na2CO3 [eqn (3)]. 56<br />
2.2 Synthesis of the complexes<br />
The nickel complex [NiPh{Ph 2PCH⋅⋅⋅C(⋅⋅⋅O)(o-OMe-C 6H 4)}(PPh 3)] 13 has been briefly<br />
described earlier 56 and was obtained in high yield by reaction of the β-keto phosphorus ylid<br />
12 with equimolar amounts of [Ni(COD)2] and PPh3 in toluene at room temperature (see<br />
Experimental). The 31 P{ 1 H} NMR spectrum of this complex should display a typical AB<br />
pattern for the trans coordinated phosphorus atoms but the small chemical shift difference<br />
between the two P resonances resulted in two intense signals at 23.6 and 23.4 ppm instead of<br />
the two doublets expected. The hydrogen atom of the C-H group α to the phosphine moiety is<br />
characterized in 1 H NMR spectroscopy by a chemical shift of 5.97 ppm, at significantly<br />
higher value than in similar complexes. 57,58 This suggested an intramolecular bonding<br />
interaction between this hydrogen atom and the ether function in ortho position to the phenyl<br />
group. A 2D NOESY spectrum confirmed this hypothesis by showing a spatial interaction<br />
between the hydrogen atom and the methyl hydrogens of the ether function.<br />
6