Anthony KERMAGORET - THESES ET MEMOIRES DE L'UDS
Anthony KERMAGORET - THESES ET MEMOIRES DE L'UDS
Anthony KERMAGORET - THESES ET MEMOIRES DE L'UDS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapitre III<br />
Ligands combining phosphorus and nitrogen or oxygen donor atoms allow a fine-tuning of the<br />
structural properties and of the reactivity (stoichiometric and catalytic) of their metal complexes. [1] In<br />
particular, complexes bearing diversely substituted phosphino- or phosphinito-oxazolines have been<br />
studied for their dynamic behavior and catalytic activity in a number of reactions (e.g. ethylene<br />
oligomerization, hydrogenation of ketones, ethylene/CO co-polymerization, cyclopropanation, Diels-<br />
Alder reaction and allylic substitutions). [1c,2]<br />
Since we recently found that phosphinothiazolines may display unique reactivity, [3] we became<br />
interested in comparing the ligands 2-diphenylphosphino-methyl-2-thiazoline (PNth) and 2-<br />
diphenylphosphino-methyl-2-oxazoline (PNox). When PNth and PNox were reacted with anhydrous<br />
NiCl2, red CH2Cl2 solutions were formed and their slow evaporation at 25 °C afforded red powders of<br />
[NiCl2(PNth)] (1) and [NiCl2(PNox)] (2), respectively. However, rapid precipitation from these<br />
solutions afforded green powders (see Supporting Information) and green single crystals were grown<br />
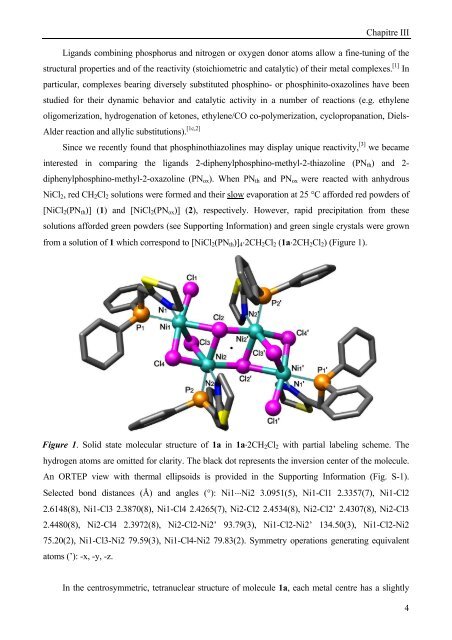
from a solution of 1 which correspond to [NiCl2(PNth)]4⋅2CH2Cl2 (1a⋅2CH2Cl2) (Figure 1).<br />
Figure 1. Solid state molecular structure of 1a in 1a⋅2CH2Cl2 with partial labeling scheme. The<br />
hydrogen atoms are omitted for clarity. The black dot represents the inversion center of the molecule.<br />
An ORTEP view with thermal ellipsoids is provided in the Supporting Information (Fig. S-1).<br />
Selected bond distances (Å) and angles (°): Ni1⋅⋅⋅Ni2 3.0951(5), Ni1-Cl1 2.3357(7), Ni1-Cl2<br />
2.6148(8), Ni1-Cl3 2.3870(8), Ni1-Cl4 2.4265(7), Ni2-Cl2 2.4534(8), Ni2-Cl2’ 2.4307(8), Ni2-Cl3<br />
2.4480(8), Ni2-Cl4 2.3972(8), Ni2-Cl2-Ni2’ 93.79(3), Ni1-Cl2-Ni2’ 134.50(3), Ni1-Cl2-Ni2<br />
75.20(2), Ni1-Cl3-Ni2 79.59(3), Ni1-Cl4-Ni2 79.83(2). Symmetry operations generating equivalent<br />
atoms (’): -x, -y, -z.<br />
In the centrosymmetric, tetranuclear structure of molecule 1a, each metal centre has a slightly<br />
4