Kinetic Analysis and Characterization of Epoxy Resins ... - FedOA
Kinetic Analysis and Characterization of Epoxy Resins ... - FedOA
Kinetic Analysis and Characterization of Epoxy Resins ... - FedOA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Results <strong>and</strong> Discussion 108<br />
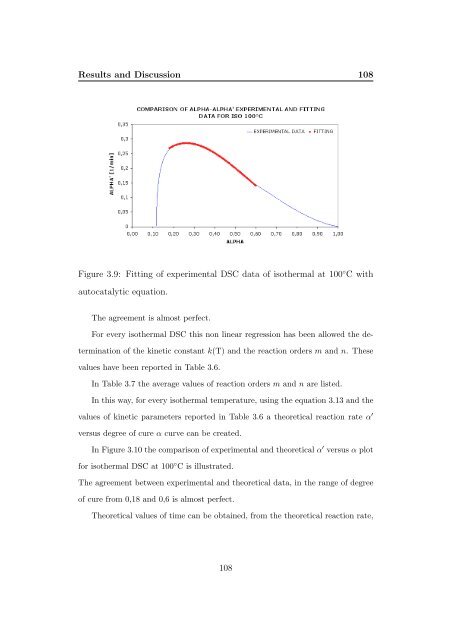
Figure 3.9: Fitting <strong>of</strong> experimental DSC data <strong>of</strong> isothermal at 100 ◦ Cwith<br />
autocatalytic equation.<br />
The agreement is almost perfect.<br />
For every isothermal DSC this non linear regression has been allowed the determination<br />
<strong>of</strong> the kinetic constant k(T) <strong>and</strong> the reaction orders m <strong>and</strong> n. These<br />
values have been reported in Table 3.6.<br />
In Table 3.7 the average values <strong>of</strong> reaction orders m <strong>and</strong> n are listed.<br />
In this way, for every isothermal temperature, using the equation 3.13 <strong>and</strong> the<br />
values <strong>of</strong> kinetic parameters reported in Table 3.6 a theoretical reaction rate α ′<br />
versus degree <strong>of</strong> cure α curve can be created.<br />
In Figure 3.10 the comparison <strong>of</strong> experimental <strong>and</strong> theoretical α ′ versus α plot<br />
for isothermal DSC at 100 ◦ C is illustrated.<br />
The agreement between experimental <strong>and</strong> theoretical data, in the range <strong>of</strong> degree<br />
<strong>of</strong> cure from 0,18 <strong>and</strong> 0,6 is almost perfect.<br />
Theoretical values <strong>of</strong> time can be obtained, from the theoretical reaction rate,<br />
108