Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
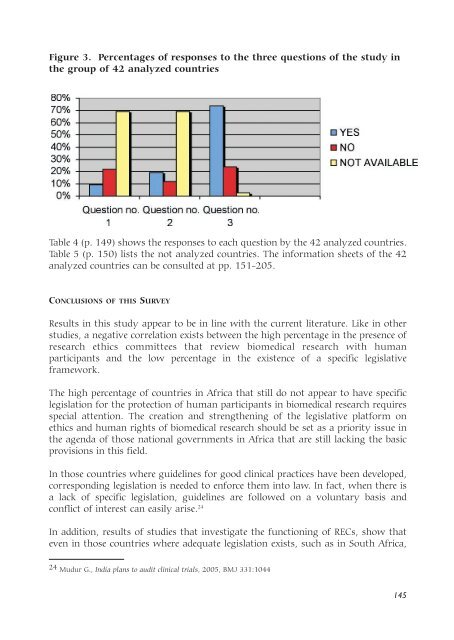
Figure 3. Percentages of responses to the three questions of the study <strong>in</strong><br />
the group of 42 analyzed countries<br />
Table 4 (p. 149) shows the responses to each question by the 42 analyzed countries.<br />
Table 5 (p. 150) lists the not analyzed countries. The <strong>in</strong>formation sheets of the 42<br />
analyzed countries can be consulted at pp. 151-205.<br />
CONCLUSIONS OF THIS SURVEY<br />
Results <strong>in</strong> this study appear to be <strong>in</strong> l<strong>in</strong>e with the current literature. Like <strong>in</strong> other<br />
studies, a negative correlation exists between the high percentage <strong>in</strong> the presence of<br />
research ethics committees that review biomedical research with human<br />
participants and the low percentage <strong>in</strong> the existence of a specific legislative<br />
framework.<br />
The high percentage of countries <strong>in</strong> Africa that still do not appear to have specific<br />
legislation for the protection of human participants <strong>in</strong> biomedical research requires<br />
special attention. The creation and strengthen<strong>in</strong>g of the legislative platform on<br />
ethics and human rights of biomedical research should be set as a priority issue <strong>in</strong><br />
the agenda of those national governments <strong>in</strong> Africa that are still lack<strong>in</strong>g the basic<br />
provisions <strong>in</strong> this field.<br />
In those countries where guidel<strong>in</strong>es for good cl<strong>in</strong>ical practices have been developed,<br />
correspond<strong>in</strong>g legislation is needed to enforce them <strong>in</strong>to law. In fact, when there is<br />
a lack of specific legislation, guidel<strong>in</strong>es are followed on a voluntary basis and<br />
conflict of <strong>in</strong>terest can easily arise. 24<br />
In addition, results of studies that <strong>in</strong>vestigate the function<strong>in</strong>g of RECs, show that<br />
even <strong>in</strong> those countries where adequate legislation exists, such as <strong>in</strong> South Africa,<br />
24 Mudur G., India plans to audit cl<strong>in</strong>ical trials, 2005, BMJ 331:1044<br />
145