Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
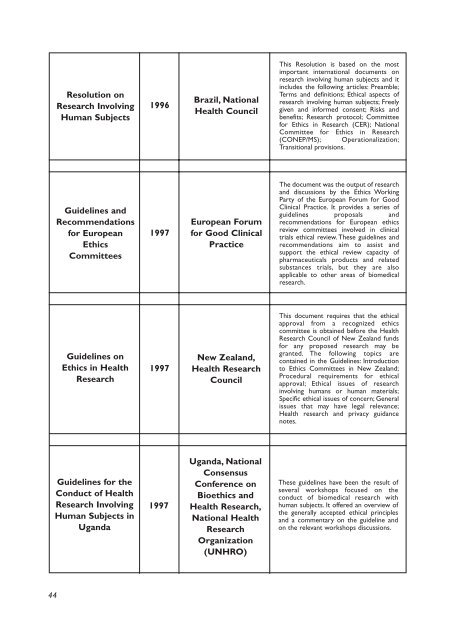
Resolution on<br />
<strong>Research</strong> Involv<strong>in</strong>g<br />
Human Subjects<br />
1996<br />
Brazil, National<br />
Health Council<br />
This Resolution is based on the most<br />
important <strong>in</strong>ternational documents on<br />
research <strong>in</strong>volv<strong>in</strong>g human subjects and it<br />
<strong>in</strong>cludes the follow<strong>in</strong>g articles: Preamble;<br />
Terms and def<strong>in</strong>itions; Ethical aspects of<br />
research <strong>in</strong>volv<strong>in</strong>g human subjects; Freely<br />
given and <strong>in</strong>formed consent; Risks and<br />
benefits; <strong>Research</strong> protocol; Committee<br />
for Ethics <strong>in</strong> <strong>Research</strong> (CER); National<br />
Committee for Ethics <strong>in</strong> <strong>Research</strong><br />
(CONEP/MS); Operationalization;<br />
Transitional provisions.<br />
Guidel<strong>in</strong>es and<br />
Recommendations<br />
for European<br />
Ethics<br />
Committees<br />
1997<br />
European Forum<br />
for Good Cl<strong>in</strong>ical<br />
Practice<br />
The document was the output of research<br />
and discussions by the Ethics Work<strong>in</strong>g<br />
Party of the European Forum for Good<br />
Cl<strong>in</strong>ical Practice. It provides a series of<br />
guidel<strong>in</strong>es proposals and<br />
recommendations for European ethics<br />
review committees <strong>in</strong>volved <strong>in</strong> cl<strong>in</strong>ical<br />
trials ethical review. These guidel<strong>in</strong>es and<br />
recommendations aim to assist and<br />
support the ethical review capacity of<br />
pharmaceuticals products and related<br />
substances trials, but they are also<br />
applicable to other areas of biomedical<br />
research.<br />
Guidel<strong>in</strong>es on<br />
Ethics <strong>in</strong> Health<br />
<strong>Research</strong><br />
1997<br />
New Zealand,<br />
Health <strong>Research</strong><br />
Council<br />
This document requires that the ethical<br />
approval from a recognized ethics<br />
committee is obta<strong>in</strong>ed before the Health<br />
<strong>Research</strong> Council of New Zealand funds<br />
for any proposed research may be<br />
granted. The follow<strong>in</strong>g topics are<br />
conta<strong>in</strong>ed <strong>in</strong> the Guidel<strong>in</strong>es: Introduction<br />
to Ethics Committees <strong>in</strong> New Zealand;<br />
Procedural requirements for ethical<br />
approval; Ethical issues of research<br />
<strong>in</strong>volv<strong>in</strong>g humans or human materials;<br />
Specific ethical issues of concern; General<br />
issues that may have legal relevance;<br />
Health research and privacy guidance<br />
notes.<br />
Guidel<strong>in</strong>es for the<br />
Conduct of Health<br />
<strong>Research</strong> Involv<strong>in</strong>g<br />
Human Subjects <strong>in</strong><br />
Uganda<br />
1997<br />
Uganda, National<br />
Consensus<br />
Conference on<br />
Bioethics and<br />
Health <strong>Research</strong>,<br />
National Health<br />
<strong>Research</strong><br />
Organization<br />
(UNHRO)<br />
These guidel<strong>in</strong>es have been the result of<br />
several workshops focused on the<br />
conduct of biomedical research with<br />
human subjects. It offered an overview of<br />
the generally accepted ethical pr<strong>in</strong>ciples<br />
and a commentary on the guidel<strong>in</strong>e and<br />
on the relevant workshops discussions.<br />
44