Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
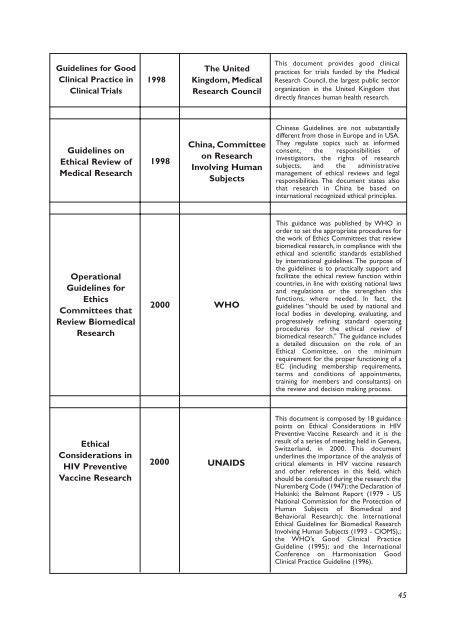
Guidel<strong>in</strong>es for Good<br />
Cl<strong>in</strong>ical Practice <strong>in</strong><br />
Cl<strong>in</strong>ical Trials<br />
1998<br />
The United<br />
K<strong>in</strong>gdom, Medical<br />
<strong>Research</strong> Council<br />
This document provides good cl<strong>in</strong>ical<br />
practices for trials funded by the Medical<br />
<strong>Research</strong> Council, the largest public sector<br />
organization <strong>in</strong> the United K<strong>in</strong>gdom that<br />
directly f<strong>in</strong>ances human health research.<br />
Guidel<strong>in</strong>es on<br />
Ethical Review of<br />
Medical <strong>Research</strong><br />
1998<br />
Ch<strong>in</strong>a, Committee<br />
on <strong>Research</strong><br />
Involv<strong>in</strong>g Human<br />
Subjects<br />
Ch<strong>in</strong>ese Guidel<strong>in</strong>es are not substantially<br />
different from those <strong>in</strong> Europe and <strong>in</strong> USA.<br />
They regulate topics such as <strong>in</strong>formed<br />
consent, the responsibilities of<br />
<strong>in</strong>vestigators, the rights of research<br />
subjects, and the adm<strong>in</strong>istrative<br />
management of ethical reviews and legal<br />
responsibilities. The document states also<br />
that research <strong>in</strong> Ch<strong>in</strong>a be based on<br />
<strong>in</strong>ternational recognized ethical pr<strong>in</strong>ciples.<br />
Operational<br />
Guidel<strong>in</strong>es for<br />
Ethics<br />
Committees that<br />
Review <strong>Biomedical</strong><br />
<strong>Research</strong><br />
2000<br />
WHO<br />
This guidance was published by WHO <strong>in</strong><br />
order to set the appropriate procedures for<br />
the work of Ethics Committees that review<br />
biomedical research, <strong>in</strong> compliance with the<br />
ethical and scientific standards established<br />
by <strong>in</strong>ternational guidel<strong>in</strong>es. The purpose of<br />
the guidel<strong>in</strong>es is to practically support and<br />
facilitate the ethical review function with<strong>in</strong><br />
countries, <strong>in</strong> l<strong>in</strong>e with exist<strong>in</strong>g national laws<br />
and regulations or the strengthen this<br />
functions, where needed. In fact, the<br />
guidel<strong>in</strong>es “should be used by national and<br />
local bodies <strong>in</strong> develop<strong>in</strong>g, evaluat<strong>in</strong>g, and<br />
progressively ref<strong>in</strong><strong>in</strong>g standard operat<strong>in</strong>g<br />
procedures for the ethical review of<br />
biomedical research.” The guidance <strong>in</strong>cludes<br />
a detailed discussion on the role of an<br />
Ethical Committee, on the m<strong>in</strong>imum<br />
requirement for the proper function<strong>in</strong>g of a<br />
EC (<strong>in</strong>clud<strong>in</strong>g membership requirements,<br />
terms and conditions of appo<strong>in</strong>tments,<br />
tra<strong>in</strong><strong>in</strong>g for members and consultants) on<br />
the review and decision mak<strong>in</strong>g process.<br />
Ethical<br />
Considerations <strong>in</strong><br />
HIV Preventive<br />
Vacc<strong>in</strong>e <strong>Research</strong><br />
2000<br />
UNAIDS<br />
This document is composed by 18 guidance<br />
po<strong>in</strong>ts on Ethical Considerations <strong>in</strong> HIV<br />
Preventive Vacc<strong>in</strong>e <strong>Research</strong> and it is the<br />
result of a series of meet<strong>in</strong>g held <strong>in</strong> Geneva,<br />
Switzerland, <strong>in</strong> 2000. This document<br />
underl<strong>in</strong>es the importance of the analysis of<br />
critical elements <strong>in</strong> HIV vacc<strong>in</strong>e research<br />
and other references <strong>in</strong> this field, which<br />
should be consulted dur<strong>in</strong>g the research: the<br />
Nuremberg Code (1947); the Declaration of<br />
Hels<strong>in</strong>ki; the Belmont Report (1979 - US<br />
National Commission for the Protection of<br />
Human Subjects of <strong>Biomedical</strong> and<br />
Behavioral <strong>Research</strong>); the International<br />
Ethical Guidel<strong>in</strong>es for <strong>Biomedical</strong> <strong>Research</strong><br />
Involv<strong>in</strong>g Human Subjects (1993 - CIOMS),;<br />
the WHO’s Good Cl<strong>in</strong>ical Practice<br />
Guidel<strong>in</strong>e (1995); and the International<br />
Conference on Harmonisation Good<br />
Cl<strong>in</strong>ical Practice Guidel<strong>in</strong>e (1996).<br />
45