Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
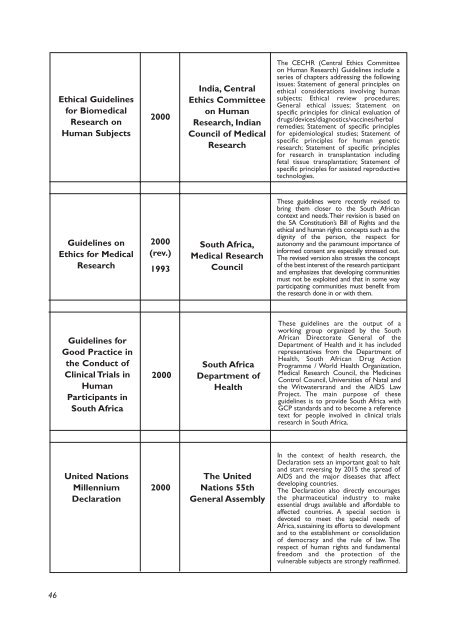
Ethical Guidel<strong>in</strong>es<br />
for <strong>Biomedical</strong><br />
<strong>Research</strong> on<br />
Human Subjects<br />
2000<br />
India, Central<br />
Ethics Committee<br />
on Human<br />
<strong>Research</strong>, Indian<br />
Council of Medical<br />
<strong>Research</strong><br />
The CECHR (Central Ethics Committee<br />
on Human <strong>Research</strong>) Guidel<strong>in</strong>es <strong>in</strong>clude a<br />
series of chapters address<strong>in</strong>g the follow<strong>in</strong>g<br />
issues: Statement of general pr<strong>in</strong>ciples on<br />
ethical considerations <strong>in</strong>volv<strong>in</strong>g human<br />
subjects; Ethical review procedures;<br />
General ethical issues; Statement on<br />
specific pr<strong>in</strong>ciples for cl<strong>in</strong>ical evaluation of<br />
drugs/devices/diagnostics/vacc<strong>in</strong>es/herbal<br />
remedies; Statement of specific pr<strong>in</strong>ciples<br />
for epidemiological studies; Statement of<br />
specific pr<strong>in</strong>ciples for human genetic<br />
research; Statement of specific pr<strong>in</strong>ciples<br />
for research <strong>in</strong> transplantation <strong>in</strong>clud<strong>in</strong>g<br />
fetal tissue transplantation; Statement of<br />
specific pr<strong>in</strong>ciples for assisted reproductive<br />
technologies.<br />
Guidel<strong>in</strong>es on<br />
Ethics for Medical<br />
<strong>Research</strong><br />
2000<br />
(rev.)<br />
1993<br />
South Africa,<br />
Medical <strong>Research</strong><br />
Council<br />
These guidel<strong>in</strong>es were recently revised to<br />
br<strong>in</strong>g them closer to the South African<br />
context and needs.Their revision is based on<br />
the SA Constitution’s Bill of Rights and the<br />
ethical and human rights concepts such as the<br />
dignity of the person, the respect for<br />
autonomy and the paramount importance of<br />
<strong>in</strong>formed consent are especially stressed out.<br />
The revised version also stresses the concept<br />
of the best <strong>in</strong>terest of the research participant<br />
and emphasizes that develop<strong>in</strong>g communities<br />
must not be exploited and that <strong>in</strong> some way<br />
participat<strong>in</strong>g communities must benefit from<br />
the research done <strong>in</strong> or with them.<br />
Guidel<strong>in</strong>es for<br />
Good Practice <strong>in</strong><br />
the Conduct of<br />
Cl<strong>in</strong>ical Trials <strong>in</strong><br />
Human<br />
Participants <strong>in</strong><br />
South Africa<br />
2000<br />
South Africa<br />
Department of<br />
Health<br />
These guidel<strong>in</strong>es are the output of a<br />
work<strong>in</strong>g group organized by the South<br />
African Directorate General of the<br />
Department of Health and it has <strong>in</strong>cluded<br />
representatives from the Department of<br />
Health, South African Drug Action<br />
Programme / World Health Organization,<br />
Medical <strong>Research</strong> Council, the Medic<strong>in</strong>es<br />
Control Council, Universities of Natal and<br />
the Witwatersrand and the AIDS Law<br />
Project. The ma<strong>in</strong> purpose of these<br />
guidel<strong>in</strong>es is to provide South Africa with<br />
GCP standards and to become a reference<br />
text for people <strong>in</strong>volved <strong>in</strong> cl<strong>in</strong>ical trials<br />
research <strong>in</strong> South Africa.<br />
United Nations<br />
Millennium<br />
Declaration<br />
2000<br />
The United<br />
Nations 55th<br />
General Assembly<br />
In the context of health research, the<br />
Declaration sets an important goal: to halt<br />
and start revers<strong>in</strong>g by 2015 the spread of<br />
AIDS and the major diseases that affect<br />
develop<strong>in</strong>g countries.<br />
The Declaration also directly encourages<br />
the pharmaceutical <strong>in</strong>dustry to make<br />
essential drugs available and affordable to<br />
affected countries. A special section is<br />
devoted to meet the special needs of<br />
Africa, susta<strong>in</strong><strong>in</strong>g its efforts to development<br />
and to the establishment or consolidation<br />
of democracy and the rule of law. The<br />
respect of human rights and fundamental<br />
freedom and the protection of the<br />
vulnerable subjects are strongly reaffirmed.<br />
46