Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Biomedical Research in Developing Countries - UNICRI
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
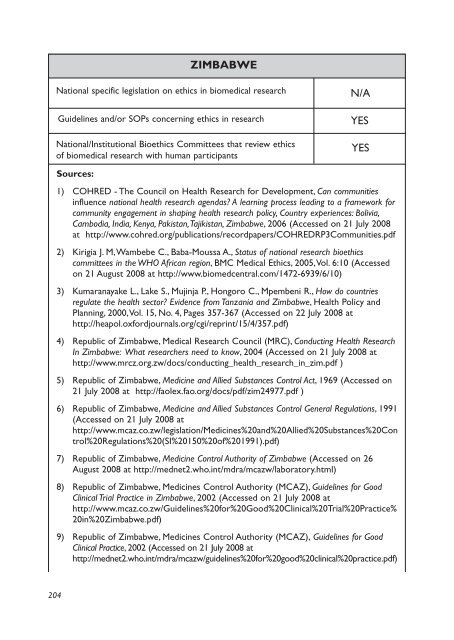
ZIMBABWE<br />
National specific legislation on ethics <strong>in</strong> biomedical research<br />
Guidel<strong>in</strong>es and/or SOPs concern<strong>in</strong>g ethics <strong>in</strong> research<br />
National/Institutional Bioethics Committees that review ethics<br />
of biomedical research with human participants<br />
N/A<br />
YES<br />
YES<br />
Sources:<br />
1) COHRED - The Council on Health <strong>Research</strong> for Development, Can communities<br />
<strong>in</strong>fluence national health research agendas? A learn<strong>in</strong>g process lead<strong>in</strong>g to a framework for<br />
community engagement <strong>in</strong> shap<strong>in</strong>g health research policy, Country experiences: Bolivia,<br />
Cambodia, India, Kenya, Pakistan,Tajikistan, Zimbabwe, 2006 (Accessed on 21 July 2008<br />
at http://www.cohred.org/publications/recordpapers/COHREDRP3Communities.pdf<br />
2) Kirigia J. M,Wambebe C., Baba-Moussa A., Status of national research bioethics<br />
committees <strong>in</strong> the WHO African region, BMC Medical Ethics, 2005,Vol. 6:10 (Accessed<br />
on 21 August 2008 at http://www.biomedcentral.com/1472-6939/6/10)<br />
3) Kumaranayake L., Lake S., Muj<strong>in</strong>ja P., Hongoro C., Mpembeni R., How do countries<br />
regulate the health sector? Evidence from Tanzania and Zimbabwe, Health Policy and<br />
Plann<strong>in</strong>g, 2000,Vol. 15, No. 4, Pages 357-367 (Accessed on 22 July 2008 at<br />
http://heapol.oxfordjournals.org/cgi/repr<strong>in</strong>t/15/4/357.pdf)<br />
4) Republic of Zimbabwe, Medical <strong>Research</strong> Council (MRC), Conduct<strong>in</strong>g Health <strong>Research</strong><br />
In Zimbabwe: What researchers need to know, 2004 (Accessed on 21 July 2008 at<br />
http://www.mrcz.org.zw/docs/conduct<strong>in</strong>g_health_research_<strong>in</strong>_zim.pdf )<br />
5) Republic of Zimbabwe, Medic<strong>in</strong>e and Allied Substances Control Act, 1969 (Accessed on<br />
21 July 2008 at http://faolex.fao.org/docs/pdf/zim24977.pdf )<br />
6) Republic of Zimbabwe, Medic<strong>in</strong>e and Allied Substances Control General Regulations, 1991<br />
(Accessed on 21 July 2008 at<br />
http://www.mcaz.co.zw/legislation/Medic<strong>in</strong>es%20and%20Allied%20Substances%20Con<br />
trol%20Regulations%20(SI%20150%20of%201991).pdf)<br />
7) Republic of Zimbabwe, Medic<strong>in</strong>e Control Authority of Zimbabwe (Accessed on 26<br />
August 2008 at http://mednet2.who.<strong>in</strong>t/mdra/mcazw/laboratory.html)<br />
8) Republic of Zimbabwe, Medic<strong>in</strong>es Control Authority (MCAZ), Guidel<strong>in</strong>es for Good<br />
Cl<strong>in</strong>ical Trial Practice <strong>in</strong> Zimbabwe, 2002 (Accessed on 21 July 2008 at<br />
http://www.mcaz.co.zw/Guidel<strong>in</strong>es%20for%20Good%20Cl<strong>in</strong>ical%20Trial%20Practice%<br />
20<strong>in</strong>%20Zimbabwe.pdf)<br />
9) Republic of Zimbabwe, Medic<strong>in</strong>es Control Authority (MCAZ), Guidel<strong>in</strong>es for Good<br />
Cl<strong>in</strong>ical Practice, 2002 (Accessed on 21 July 2008 at<br />
http://mednet2.who.<strong>in</strong>t/mdra/mcazw/guidel<strong>in</strong>es%20for%20good%20cl<strong>in</strong>ical%20practice.pdf)<br />
204