Principles of terrestrial ecosystem ecology.pdf
Principles of terrestrial ecosystem ecology.pdf
Principles of terrestrial ecosystem ecology.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
202 9. Terrestrial Nutrient Cycling<br />
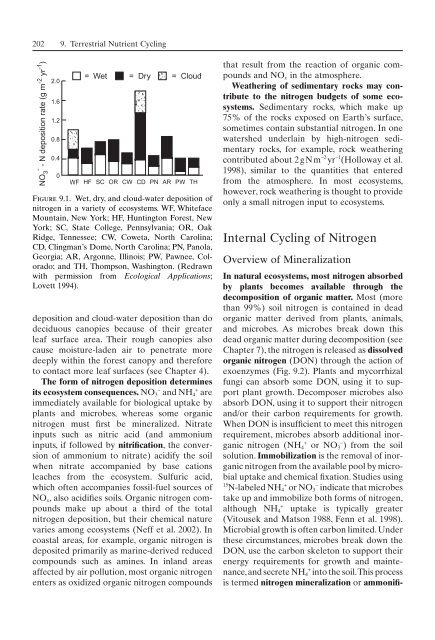
- - N deposition rate (g m -2 yr -1 )<br />
NO 3<br />
2.0<br />
1.6<br />
1.2<br />
0.8<br />
0.4<br />
0<br />
= Wet = Dry = Cloud<br />
WF HF SC OR CW CD PN AR PW TH<br />
Figure 9.1. Wet, dry, and cloud-water deposition <strong>of</strong><br />
nitrogen in a variety <strong>of</strong> <strong>ecosystem</strong>s. WF, Whiteface<br />
Mountain, New York; HF, Huntington Forest, New<br />
York; SC, State College, Pennsylvania; OR, Oak<br />
Ridge, Tennessee; CW, Coweta, North Carolina;<br />
CD, Clingman’s Dome, North Carolina; PN, Panola,<br />
Georgia; AR, Argonne, Illinois; PW, Pawnee, Colorado;<br />
and TH, Thompson, Washington. (Redrawn<br />
with permission from Ecological Applications;<br />
Lovett 1994).<br />
deposition and cloud-water deposition than do<br />
deciduous canopies because <strong>of</strong> their greater<br />
leaf surface area. Their rough canopies also<br />
cause moisture-laden air to penetrate more<br />
deeply within the forest canopy and therefore<br />
to contact more leaf surfaces (see Chapter 4).<br />
The form <strong>of</strong> nitrogen deposition determines<br />
its <strong>ecosystem</strong> consequences. NO3 - and NH4 + are<br />
immediately available for biological uptake by<br />
plants and microbes, whereas some organic<br />
nitrogen must first be mineralized. Nitrate<br />
inputs such as nitric acid (and ammonium<br />
inputs, if followed by nitrification, the conversion<br />
<strong>of</strong> ammonium to nitrate) acidify the soil<br />
when nitrate accompanied by base cations<br />
leaches from the <strong>ecosystem</strong>. Sulfuric acid,<br />
which <strong>of</strong>ten accompanies fossil-fuel sources <strong>of</strong><br />
NOx, also acidifies soils. Organic nitrogen compounds<br />
make up about a third <strong>of</strong> the total<br />
nitrogen deposition, but their chemical nature<br />
varies among <strong>ecosystem</strong>s (Neff et al. 2002). In<br />
coastal areas, for example, organic nitrogen is<br />
deposited primarily as marine-derived reduced<br />
compounds such as amines. In inland areas<br />
affected by air pollution, most organic nitrogen<br />
enters as oxidized organic nitrogen compounds<br />
that result from the reaction <strong>of</strong> organic compounds<br />
and NOx in the atmosphere.<br />
Weathering <strong>of</strong> sedimentary rocks may contribute<br />
to the nitrogen budgets <strong>of</strong> some <strong>ecosystem</strong>s.<br />
Sedimentary rocks, which make up<br />
75% <strong>of</strong> the rocks exposed on Earth’s surface,<br />
sometimes contain substantial nitrogen. In one<br />
watershed underlain by high-nitrogen sedimentary<br />
rocks, for example, rock weathering<br />
contributed about 2gNm -2 yr -1 (Holloway et al.<br />
1998), similar to the quantities that entered<br />
from the atmosphere. In most <strong>ecosystem</strong>s,<br />
however, rock weathering is thought to provide<br />
only a small nitrogen input to <strong>ecosystem</strong>s.<br />
Internal Cycling <strong>of</strong> Nitrogen<br />
Overview <strong>of</strong> Mineralization<br />
In natural <strong>ecosystem</strong>s, most nitrogen absorbed<br />
by plants becomes available through the<br />
decomposition <strong>of</strong> organic matter. Most (more<br />
than 99%) soil nitrogen is contained in dead<br />
organic matter derived from plants, animals,<br />
and microbes. As microbes break down this<br />
dead organic matter during decomposition (see<br />
Chapter 7), the nitrogen is released as dissolved<br />
organic nitrogen (DON) through the action <strong>of</strong><br />
exoenzymes (Fig. 9.2). Plants and mycorrhizal<br />
fungi can absorb some DON, using it to support<br />
plant growth. Decomposer microbes also<br />
absorb DON, using it to support their nitrogen<br />
and/or their carbon requirements for growth.<br />
When DON is insufficient to meet this nitrogen<br />
requirement, microbes absorb additional inorganic<br />
nitrogen (NH4 + or NO3 - ) from the soil<br />
solution. Immobilization is the removal <strong>of</strong> inorganic<br />
nitrogen from the available pool by microbial<br />
uptake and chemical fixation. Studies using<br />
15 N-labeled NH4 + or NO3 - indicate that microbes<br />
take up and immobilize both forms <strong>of</strong> nitrogen,<br />
although NH4 + uptake is typically greater<br />
(Vitousek and Matson 1988, Fenn et al. 1998).<br />
Microbial growth is <strong>of</strong>ten carbon limited. Under<br />
these circumstances, microbes break down the<br />
DON, use the carbon skeleton to support their<br />
energy requirements for growth and maintenance,and<br />
secrete NH4 + into the soil.This process<br />
is termed nitrogen mineralization or ammonifi-