Principles of terrestrial ecosystem ecology.pdf
Principles of terrestrial ecosystem ecology.pdf
Principles of terrestrial ecosystem ecology.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
218 9. Terrestrial Nutrient Cycling<br />
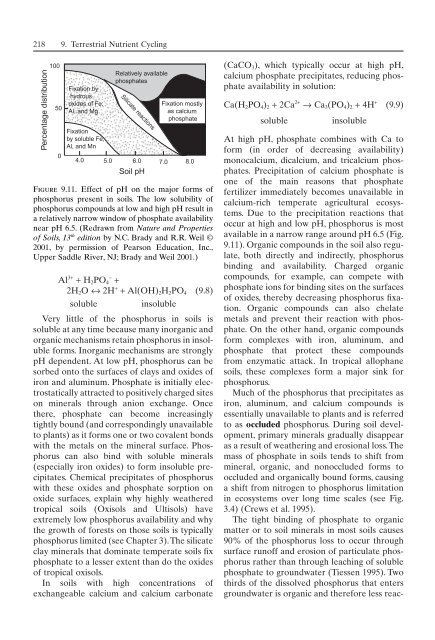
Percentage distribution<br />
100<br />
50<br />
0<br />
Fixation by<br />
hydrous<br />
oxides <strong>of</strong> Fe,<br />
Al, and Mg<br />
Fixation<br />
by soluble Fe,<br />
Al, and Mn<br />
Relatively available<br />
phosphates<br />
Silicate reactions<br />
4.0 5.0 6.0<br />
Soil pH<br />
7.0 8.0<br />
Al 3+ + H2PO4 - +<br />
2H2O ´ 2H + + Al(OH)2H2PO4 (9.8)<br />
soluble insoluble<br />
Fixation mostly<br />
as calcium<br />
phosphate<br />
Figure 9.11. Effect <strong>of</strong> pH on the major forms <strong>of</strong><br />
phosphorus present in soils. The low solubility <strong>of</strong><br />
phosphorus compounds at low and high pH result in<br />
a relatively narrow window <strong>of</strong> phosphate availability<br />
near pH 6.5. (Redrawn from Nature and Properties<br />
<strong>of</strong> Soils, 13 th edition by N.C. Brady and R.R. Weil ©<br />
2001, by permission <strong>of</strong> Pearson Education, Inc.,<br />
Upper Saddle River, NJ; Brady and Weil 2001.)<br />
Very little <strong>of</strong> the phosphorus in soils is<br />
soluble at any time because many inorganic and<br />
organic mechanisms retain phosphorus in insoluble<br />
forms. Inorganic mechanisms are strongly<br />
pH dependent. At low pH, phosphorus can be<br />
sorbed onto the surfaces <strong>of</strong> clays and oxides <strong>of</strong><br />
iron and aluminum. Phosphate is initially electrostatically<br />
attracted to positively charged sites<br />
on minerals through anion exchange. Once<br />
there, phosphate can become increasingly<br />
tightly bound (and correspondingly unavailable<br />
to plants) as it forms one or two covalent bonds<br />
with the metals on the mineral surface. Phosphorus<br />
can also bind with soluble minerals<br />
(especially iron oxides) to form insoluble precipitates.<br />
Chemical precipitates <strong>of</strong> phosphorus<br />
with these oxides and phosphate sorption on<br />
oxide surfaces, explain why highly weathered<br />
tropical soils (Oxisols and Ultisols) have<br />
extremely low phosphorus availability and why<br />
the growth <strong>of</strong> forests on those soils is typically<br />
phosphorus limited (see Chapter 3).The silicate<br />
clay minerals that dominate temperate soils fix<br />
phosphate to a lesser extent than do the oxides<br />
<strong>of</strong> tropical oxisols.<br />
In soils with high concentrations <strong>of</strong><br />
exchangeable calcium and calcium carbonate<br />
(CaCO3), which typically occur at high pH,<br />
calcium phosphate precipitates, reducing phosphate<br />
availability in solution:<br />
Ca(H2PO4)2 + 2Ca2+ Æ Ca3(PO4)2 + 4H + (9.9)<br />
soluble insoluble<br />
At high pH, phosphate combines with Ca to<br />
form (in order <strong>of</strong> decreasing availability)<br />
monocalcium, dicalcium, and tricalcium phosphates.<br />
Precipitation <strong>of</strong> calcium phosphate is<br />
one <strong>of</strong> the main reasons that phosphate<br />
fertilizer immediately becomes unavailable in<br />
calcium-rich temperate agricultural <strong>ecosystem</strong>s.<br />
Due to the precipitation reactions that<br />
occur at high and low pH, phosphorus is most<br />
available in a narrow range around pH 6.5 (Fig.<br />
9.11). Organic compounds in the soil also regulate,<br />
both directly and indirectly, phosphorus<br />
binding and availability. Charged organic<br />
compounds, for example, can compete with<br />
phosphate ions for binding sites on the surfaces<br />
<strong>of</strong> oxides, thereby decreasing phosphorus fixation.<br />
Organic compounds can also chelate<br />
metals and prevent their reaction with phosphate.<br />
On the other hand, organic compounds<br />
form complexes with iron, aluminum, and<br />
phosphate that protect these compounds<br />
from enzymatic attack. In tropical allophane<br />
soils, these complexes form a major sink for<br />
phosphorus.<br />
Much <strong>of</strong> the phosphorus that precipitates as<br />
iron, aluminum, and calcium compounds is<br />
essentially unavailable to plants and is referred<br />
to as occluded phosphorus. During soil development,<br />
primary minerals gradually disappear<br />
as a result <strong>of</strong> weathering and erosional loss.The<br />
mass <strong>of</strong> phosphate in soils tends to shift from<br />
mineral, organic, and nonoccluded forms to<br />
occluded and organically bound forms, causing<br />
a shift from nitrogen to phosphorus limitation<br />
in <strong>ecosystem</strong>s over long time scales (see Fig.<br />
3.4) (Crews et al. 1995).<br />
The tight binding <strong>of</strong> phosphate to organic<br />
matter or to soil minerals in most soils causes<br />
90% <strong>of</strong> the phosphorus loss to occur through<br />
surface run<strong>of</strong>f and erosion <strong>of</strong> particulate phosphorus<br />
rather than through leaching <strong>of</strong> soluble<br />
phosphate to groundwater (Tiessen 1995). Two<br />
thirds <strong>of</strong> the dissolved phosphorus that enters<br />
groundwater is organic and therefore less reac-