Principles of terrestrial ecosystem ecology.pdf
Principles of terrestrial ecosystem ecology.pdf
Principles of terrestrial ecosystem ecology.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
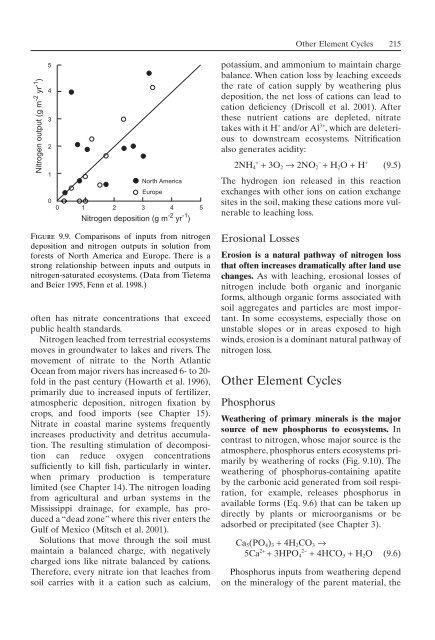
Nitrogen output (g m -2 yr -1 )<br />
5<br />
4<br />
3<br />
2<br />
1<br />
0<br />
North America<br />
Europe<br />
0 1 2 3 4<br />
Nitrogen deposition (g m -2 yr -1 )<br />
Figure 9.9. Comparisons <strong>of</strong> inputs from nitrogen<br />
deposition and nitrogen outputs in solution from<br />
forests <strong>of</strong> North America and Europe. There is a<br />
strong relationship between inputs and outputs in<br />
nitrogen-saturated <strong>ecosystem</strong>s. (Data from Tietema<br />
and Beier 1995, Fenn et al. 1998.)<br />
<strong>of</strong>ten has nitrate concentrations that exceed<br />
public health standards.<br />
Nitrogen leached from <strong>terrestrial</strong> <strong>ecosystem</strong>s<br />
moves in groundwater to lakes and rivers. The<br />
movement <strong>of</strong> nitrate to the North Atlantic<br />
Ocean from major rivers has increased 6- to 20fold<br />
in the past century (Howarth et al. 1996),<br />
primarily due to increased inputs <strong>of</strong> fertilizer,<br />
atmospheric deposition, nitrogen fixation by<br />
crops, and food imports (see Chapter 15).<br />
Nitrate in coastal marine systems frequently<br />
increases productivity and detritus accumulation.<br />
The resulting stimulation <strong>of</strong> decomposition<br />
can reduce oxygen concentrations<br />
sufficiently to kill fish, particularly in winter,<br />
when primary production is temperature<br />
limited (see Chapter 14). The nitrogen loading<br />
from agricultural and urban systems in the<br />
Mississippi drainage, for example, has produced<br />
a “dead zone” where this river enters the<br />
Gulf <strong>of</strong> Mexico (Mitsch et al. 2001).<br />
Solutions that move through the soil must<br />
maintain a balanced charge, with negatively<br />
charged ions like nitrate balanced by cations.<br />
Therefore, every nitrate ion that leaches from<br />
soil carries with it a cation such as calcium,<br />
5<br />
Other Element Cycles 215<br />
potassium, and ammonium to maintain charge<br />
balance. When cation loss by leaching exceeds<br />
the rate <strong>of</strong> cation supply by weathering plus<br />
deposition, the net loss <strong>of</strong> cations can lead to<br />
cation deficiency (Driscoll et al. 2001). After<br />
these nutrient cations are depleted, nitrate<br />
takes with it H + and/or Al 3+ , which are deleterious<br />
to downstream <strong>ecosystem</strong>s. Nitrification<br />
also generates acidity:<br />
2NH4 + + 3O2 Æ 2NO2 - + H2O + H + (9.5)<br />
The hydrogen ion released in this reaction<br />
exchanges with other ions on cation exchange<br />
sites in the soil, making these cations more vulnerable<br />
to leaching loss.<br />
Erosional Losses<br />
Erosion is a natural pathway <strong>of</strong> nitrogen loss<br />
that <strong>of</strong>ten increases dramatically after land use<br />
changes. As with leaching, erosional losses <strong>of</strong><br />
nitrogen include both organic and inorganic<br />
forms, although organic forms associated with<br />
soil aggregates and particles are most important.<br />
In some <strong>ecosystem</strong>s, especially those on<br />
unstable slopes or in areas exposed to high<br />
winds, erosion is a dominant natural pathway <strong>of</strong><br />
nitrogen loss.<br />
Other Element Cycles<br />
Phosphorus<br />
Weathering <strong>of</strong> primary minerals is the major<br />
source <strong>of</strong> new phosphorus to <strong>ecosystem</strong>s. In<br />
contrast to nitrogen, whose major source is the<br />
atmosphere, phosphorus enters <strong>ecosystem</strong>s primarily<br />
by weathering <strong>of</strong> rocks (Fig. 9.10). The<br />
weathering <strong>of</strong> phosphorus-containing apatite<br />
by the carbonic acid generated from soil respiration,<br />
for example, releases phosphorus in<br />
available forms (Eq. 9.6) that can be taken up<br />
directly by plants or microorganisms or be<br />
adsorbed or precipitated (see Chapter 3).<br />
Ca5(PO4)3 + 4H2CO3 Æ<br />
5Ca 2+ + 3HPO4 2- + 4HCO3 + H2O (9.6)<br />
Phosphorus inputs from weathering depend<br />
on the mineralogy <strong>of</strong> the parent material, the