Principles of terrestrial ecosystem ecology.pdf
Principles of terrestrial ecosystem ecology.pdf
Principles of terrestrial ecosystem ecology.pdf
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
chemical weathering through their contribution<br />
to soil acidity and their capacity to chelate ions.<br />
In the chelation process, organic acids combine<br />
with metallic ions, such as ferric iron (Fe 3+ ) and<br />
aluminum (Al 3+ ), making them soluble and<br />
mobile. Chelation lowers the concentration <strong>of</strong><br />
inorganic ions at the mineral surface, so dissolved<br />
and primary mineral forms are no longer<br />
in equilibrium with one another. This accelerates<br />
the rate <strong>of</strong> weathering.<br />
The physical and chemical properties <strong>of</strong><br />
rock minerals determine their susceptibility<br />
to weathering and the chemical products that<br />
result. Sedimentary rocks like shale that form<br />
by chemical precipitation, for example, have<br />
more basic cations like calcium (Ca 2+ ), sodium<br />
(Na + ) and potassium (K + ) than does igneous<br />
rock and tends to produce soils with a relatively<br />
high pH and a high capacity to supply mineral<br />
cations to plants. Igneous rocks weather in the<br />
reverse order in which they crystallize during<br />
formation (Birkeland 1999). Olivine, for<br />
example, is one <strong>of</strong> the first minerals to crystallize<br />
as magma cools. It has a high energy <strong>of</strong><br />
formation and weathers easily. Feldspar forms<br />
and weathers more slowly than olivine, and<br />
quartz is one <strong>of</strong> the last minerals to form<br />
(explaining why it forms crystals) and is highly<br />
resistant to weathering (Table 3.2). Secondary<br />
minerals such as the silicate clay minerals and<br />
iron and aluminum oxides are among the most<br />
resistant minerals to weathering. Textural differences<br />
in parent material also influence the<br />
rate <strong>of</strong> chemical breakdown, with fine-grained<br />
rocks weathering more slowly than coarsegrained<br />
rocks.<br />
Warm climates promote chemical weathering<br />
because temperature speeds chemical reactions<br />
Table 3.2. Stability <strong>of</strong> common minerals under<br />
weathering conditions at Earth’s surface.<br />
Most stable Fe 3+ oxides Secondary mineral<br />
Al 3+ oxides Secondary mineral<br />
Quartz Primary mineral<br />
Clay minerals Secondary mineral<br />
K + feldspar Primary mineral<br />
Na + feldspar Primary mineral<br />
Ca 2+ feldspar Primary mineral<br />
Least stable Olivine Primary mineral<br />
Data from Press and Siever (1986).<br />
Development <strong>of</strong> Soil Pr<strong>of</strong>iles 55<br />
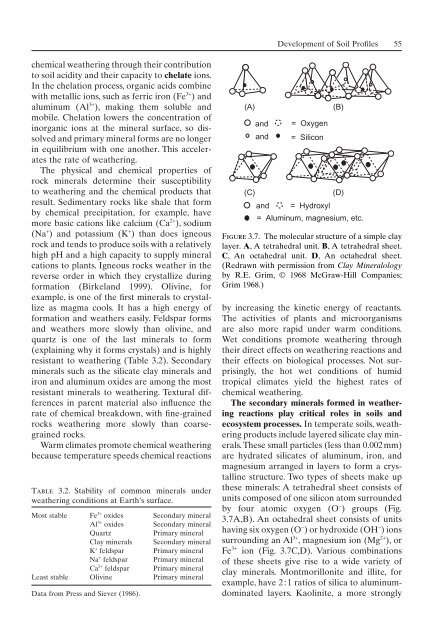
(A) (B)<br />
and<br />
and<br />
= Oxygen<br />
= Silicon<br />
(C) (D)<br />
and = Hydroxyl<br />
= Aluminum, magnesium, etc.<br />
Figure 3.7. The molecular structure <strong>of</strong> a simple clay<br />
layer. A, A tetrahedral unit. B, A tetrahedral sheet.<br />
C, An octahedral unit. D, An octahedral sheet.<br />
(Redrawn with permission from Clay Mineralology<br />
by R.E. Grim, © 1968 McGraw-Hill Companies;<br />
Grim 1968.)<br />
by increasing the kinetic energy <strong>of</strong> reactants.<br />
The activities <strong>of</strong> plants and microorganisms<br />
are also more rapid under warm conditions.<br />
Wet conditions promote weathering through<br />
their direct effects on weathering reactions and<br />
their effects on biological processes. Not surprisingly,<br />
the hot wet conditions <strong>of</strong> humid<br />
tropical climates yield the highest rates <strong>of</strong><br />
chemical weathering.<br />
The secondary minerals formed in weathering<br />
reactions play critical roles in soils and<br />
<strong>ecosystem</strong> processes. In temperate soils, weathering<br />
products include layered silicate clay minerals.<br />
These small particles (less than 0.002mm)<br />
are hydrated silicates <strong>of</strong> aluminum, iron, and<br />
magnesium arranged in layers to form a crystalline<br />
structure. Two types <strong>of</strong> sheets make up<br />
these minerals: A tetrahedral sheet consists <strong>of</strong><br />
units composed <strong>of</strong> one silicon atom surrounded<br />
by four atomic oxygen (O - ) groups (Fig.<br />
3.7A,B). An octahedral sheet consists <strong>of</strong> units<br />
having six oxygen (O - ) or hydroxide (OH - ) ions<br />
surrounding an Al 3+ , magnesium ion (Mg 2+ ), or<br />
Fe 3+ ion (Fig. 3.7C,D). Various combinations<br />
<strong>of</strong> these sheets give rise to a wide variety <strong>of</strong><br />
clay minerals. Montmorillonite and illite, for<br />
example, have 2:1 ratios <strong>of</strong> silica to aluminumdominated<br />
layers. Kaolinite, a more strongly