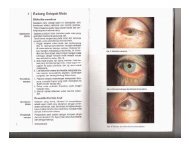
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Arginine-Rich Molecular Transporters for Drugs 159<br />
biological activity, as is the case for amphipathic, antimicrobial peptides. 19,20<br />
Increased flexibility in the backbone or the side chains could allow the guanidine<br />
headgroups to interact more effectively with negative charges on the cell surface.<br />
Using CPK models, increasing the number of methylenes between the arginines not<br />
only allows them to interact with negative charges further apart than homopolymers<br />
of arginine but, interestingly, also permits the guanidines to pack more closely and<br />
interact with putative negative charges that are closely spaced. Further experiments<br />
are needed to distinguish among these possible explanations.<br />
REFERENCES<br />
1. Mitchell, D.J. et al., Polyarginine enters cells more efficiently than other polycationic<br />
homopolymers, J. Pept. Res., 56, 318, 2000.<br />
2. Wender, P.A. et al., The design, synthesis, and evaluation of molecules that enable<br />
or enhance cellular uptake: peptoid molecular transporters, Proc. Natl. Acad. Sci.<br />
U.S.A., 97, 13003, 2000.<br />
3. Zuckermann, R. et al., Efficient method for the preparation of peptoids [oligo (Nsubstituted<br />
glycines)] by sub-nanomer solid phase synthesis, JACS, 114, 10646, 1992.<br />
4. Pons, J. et al., A constrained diketopiperazine as a new scaffold for the synthesis of<br />
peptidomimetics, Eur. J. Org. Chem., 1998, 853, 1998.<br />
5. Murphy, J.E. et al., A combinatorial approach to the discovery of efficient cationic<br />
peptoid reagents for gene delivery, Proc. Natl. Acad. Sci. U.S.A., 95, 1517, 1998.<br />
6. Simon, R.J. et al., Peptoids: a modular approach to drug discovery, Proc. Natl. Acad.<br />
Sci. U.S.A., 89, 9367, 1992.<br />
7. Kale L. et al., NAMD2: greater scalability for parallel molecular dynamics, J. Computational<br />
Physi., 151, 283, 1999.<br />
8. MacKerell, B.D. et al., All atom empirical potential for molecular modeling and<br />
dynamics studies of proteins, J. Phys. Chem., 102, 3586, 1998.<br />
9. Humphrey, W., Dalke, A., and Schulten, K., VMD — visual molecular dynamics, J.<br />
Mol. Graphics, 14, 33, 1996.<br />
10. Miller, S. et al., Proteolystic studies of homologous peptide and N-substituted glycine<br />
peptoid oligomers, Bioorg. Phys. Lett., 4, 2657, 1994.<br />
11. Tamilarasu, N., Huq, I., and Rana, T., High affinity and specific binding of HIV-1<br />
TAR RNA by a Tat-derived oligourea, JACS, 121, 1597, 1999.<br />
12. Hamy, F. et al., An inhibitor of the Tat/TAR RNA interaction that effectively sup<strong>press</strong>es<br />
HIV-1 replication, Proc. Natl. Acad. Sci. U.S.A., 94, 3548, 1997.<br />
13. Bernatowicz, M., 1H-pyrrole-1-carboxamidine hydrochloride: an attractive reagent<br />
for guanylation of amines and its application to peptide synthesis, J. Org. Chem. 57,<br />
2497, 1992.<br />
14. Kruijtzer, J. et al., Solid-phase syntheses of peptoids using Fmoc-protected N-substituted<br />
glycines: the synthesis of (retro) peptoids of Leu-enkephalin and substance<br />
P, Chem. A Eur. J., 4, 1570, 1998.<br />
15. Heizmann, G. and Felder, E.R., Synthesis of an N-3-guanidinopropylglycine (Narg)<br />
derivative as a versatile building block for solid-phase peptide and peptoid synthesis,<br />
Pept. Res., 7, 328, 1994.<br />
16. Feichtinger, K. et al., Triurethane-protected guanidines and triflyldiurethane-protected<br />
guanidines: new reagents for guanidinylation reactions, J. Org. Chem., 63, 8432, 1998.









![SISTEM SENSORY [Compatibility Mode].pdf](https://img.yumpu.com/20667975/1/190x245/sistem-sensory-compatibility-modepdf.jpg?quality=85)