Drosophila - Severo Ochoa - Universidad Autónoma de Madrid
Drosophila - Severo Ochoa - Universidad Autónoma de Madrid
Drosophila - Severo Ochoa - Universidad Autónoma de Madrid
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Jefe <strong>de</strong> Línea /<br />
Group Lea<strong>de</strong>r:<br />
Isabel Guerrero<br />
Mecanismos <strong>de</strong> señalización<br />
en el <strong>de</strong>sarrollo<br />
Signaling mechanisms<br />
in <strong>de</strong>velopment<br />
A5<br />
Publicaciones<br />
Publications<br />
Resumen <strong>de</strong> investigación<br />
Research summary<br />
Sánchez, L., Gorfinkiel, N. and Guerrero, I. (2005). Sex Determination<br />
and the Development of the Genital Disc. In: Gilbert, L: I:, Iatrou, K<br />
and Gil, S. S. (eds). Comprehensive Molecular Insect Science.<br />
Elsevier BVR. Vol I. Chapter I.<br />
Postdoctorales /<br />
Postdoctoral:<br />
Isabel Rodríguez<br />
Luis Quijada<br />
Ainhoa Callejo<br />
Aphrodite Bilioni<br />
Becarios Predoctorales /<br />
Predoctoral Fellows:<br />
Javier Sierra<br />
Elvira Benítez<br />
Alicia Rosales<br />
Técnicos <strong>de</strong> Investigación /<br />
Technical Assistance:<br />
Carmen Ibáñez<br />
Estudiantes <strong>de</strong> licenciatura /<br />
Un<strong>de</strong>rgraduated Stu<strong>de</strong>nts:<br />
Mª Jesús Delgado<br />
Biología <strong>de</strong>l Desarrollo Developmental Biology<br />
Una cuestión fundamental en biología <strong>de</strong>l <strong>de</strong>sarrollo es<br />
cómo las células precursoras <strong>de</strong> un tejido u órgano pue<strong>de</strong>n<br />
recibir e interpretar la información posicional que<br />
<strong>de</strong>terminará su <strong>de</strong>stino celular. Las moléculas <strong>de</strong> secreción<br />
perteneciente a las familias <strong>de</strong> TGF-beta, Wnt y Hedgehog<br />
(Hh) actúan como morfógenos en muchos contextos<br />
celulares, es <strong>de</strong>cir, emanan <strong>de</strong>s<strong>de</strong> focos localizados y<br />
forman gradientes extracelulares que, a su vez, <strong>de</strong>terminan<br />
el <strong>de</strong>stino celular <strong>de</strong> forma <strong>de</strong>pendiente <strong>de</strong> la<br />
concentración. Nuestro grupo está interesado en el estudio<br />
<strong>de</strong> los mecanismos moleculares <strong>de</strong> señalización <strong>de</strong> estos<br />
morfógenos. En los últimos años hemos <strong>de</strong>dicado una<br />
mayor atención a Hh, <strong>de</strong>bido a su gran importancia en<br />
enfermeda<strong>de</strong>s humanas. Así, alteraciones en la<br />
señalización <strong>de</strong> Hh dan lugar a varios tipos <strong>de</strong><br />
malformaciones en humanos, siendo causa también <strong>de</strong>l<br />
<strong>de</strong>sarrollo <strong>de</strong> tumores, tales como los carcinomas <strong>de</strong><br />
células básales (BCC) y los meduloblastomas, jugando,<br />
a<strong>de</strong>más, un papel importante en la progresión <strong>de</strong> otros tipos<br />
<strong>de</strong> tumores y en los procesos regenerativos.<br />
La proteína Hh, por ser una molécula altamente modificada<br />
por lípidos, se encuentra normalmente asociada a<br />
membranas; a pesar <strong>de</strong> ello, Hh es capaz <strong>de</strong> migrar y<br />
programar a células muy alejadas <strong>de</strong> su fuente <strong>de</strong><br />
producción. En este contexto, recientemente nuestro grupo<br />
ha i<strong>de</strong>ntificado y caracterizado el gen shifted (shf) como un<br />
nuevo gen que se requiere para la distribución <strong>de</strong> Hh fuera<br />
<strong>de</strong> la célula. Shf es un factor <strong>de</strong> secreción que podría<br />
conferir la especificad <strong>de</strong> los heparán sulfato<br />
proteoglicanos <strong>de</strong> la matriz extracelular para la retención y<br />
difusión <strong>de</strong> Hh. A<strong>de</strong>más, hemos observado que las<br />
modificaciones lipídicas <strong>de</strong> Hh se requieren para la<br />
interacción <strong>de</strong> Hh con algunos componentes <strong>de</strong> la matriz<br />
extracelular (como los Heparán Sulfato Proteoglicanos y<br />
Shf) y para la correcta interacción <strong>de</strong> Hh con su receptor<br />
Patched. Recientemente, hemos encontrado que Patched<br />
actúa como no sólo como receptor <strong>de</strong> Hh sino como un<br />
receptor <strong>de</strong> partículas lipoproteicas (LDLR). Actualmente,<br />
estamos analizando por un lado la relación que tiene el<br />
metabolismo energético con la vía <strong>de</strong> Hh y por otro el<br />
mecanismo por el que Hh unido a lipoproteínas se secreta y<br />
se mueve en el espacio extracelular.<br />
A key issue in <strong>de</strong>velopmental biology is how cells in a<br />
<strong>de</strong>veloping field acquire the positional information that will<br />
<strong>de</strong>termine their fate. Secreted signaling molecules of the<br />
TGF-beta, Wnt, and Hedgehog (Hh) families have been<br />
shown to play essential roles in cell fate specification during<br />
<strong>de</strong>velopment. In many <strong>de</strong>velopmental contexts, they act as<br />
morphogens that emanate from localized sources and form<br />
extracellular gradients, which differentially regulate cell fates<br />
in a concentration-<strong>de</strong>pen<strong>de</strong>nt manner. Our group is<br />
interested to study the function of these three morphogens<br />
in <strong>Drosophila</strong> <strong>de</strong>velopment with higher input in the molecular<br />
and cellular mechanisms of Hh signaling because its<br />
implications in human diseases. Thus, Hh pathway, which<br />
has been previously i<strong>de</strong>ntified in <strong>Drosophila</strong>, is affected in<br />
different congenital malformations and also plays a key role<br />
in <strong>de</strong>velopment and progression of various malignant<br />
tumors and in the regenerative processes.<br />
Hh is a molecule highly modified by lipids and <strong>de</strong>spite being<br />
associated with membranes, it is able to migrate and to<br />
program cells far away from its site of production. To<br />
investigate the Hh spreading mechanism, we characterized<br />
Shifted (Shf) as a new component in the <strong>Drosophila</strong> Hh<br />
pathway. We show that Shf is required for Hh stability and for<br />
lipid-modified Hh spreading. Shf is a secreted factor, which<br />
interacts both with Hh and the Heparan sulfate<br />
proteoglycans (HSPG), leading us to suggest that Shf could<br />
provi<strong>de</strong> HSPG specific for Hh. In addition, we have<br />
observed that lipid modifications on Hh are required for<br />
proper interaction with the extracellular matrix components<br />
for Hh spreading and for correct recognition of the Patched<br />
receptor. More recently, we have found that Ptc is acting also<br />
as a receptor of lipoprotein particles. Presently, we are<br />
analyzing the relationship of the lipid metabolism with the Hh<br />
pathway and also the implication of lipids and<br />
Apolipoproteins in Hh secretion and spreading.<br />
Quijada, L., Callejo, A., Torroja, C. and Guerrero, I. (2005). The<br />
Patched receptor: switching on/off the Hedgehog signaling pathway.<br />
In: Ruiz i Altaba, A (ed). Hedgehog-Gli Signaling and Human Disease.<br />
Lan<strong>de</strong>s Bioscience publishers.<br />
Gorfinkiel, N., Sierra, J., Callejo, A., Ibánez, C. and Guerrero I. (2005).<br />
The <strong>Drosophila</strong>ortholog of the human Wnt inhibitor factor Shifted<br />
controls the diffusion of lipid-modified Hedgehog. Dev Cell. 8, 241-253.<br />
Torroja, C., Gorfinkiel, N. and Guerrero, I. (2005).<br />
Mechanisms of Hedgehog gradientformation and interpretation.<br />
J Neurobiol. 64, 334-356.<br />
Callejo, A., Torroja, C., Quijada, L. and Guerrero, I. (2006).<br />
Hedgehog lipid modifications are required for Hedgehog stabilization<br />
in the extracellular matrix. Development. 133, 471-483.<br />
Callejo, A., Quijada, L. and Guerrero, I. (2006). Immunocytochemistry<br />
and <strong>de</strong>tecting tagged Hedgehog components for functional analysis.<br />
Methods in Cell Biology. The Humana Press, Inc., NJ, USA.<br />
Patentes<br />
Patents<br />
Mo<strong>de</strong>lo animal, no humano, para la i<strong>de</strong>ntificación <strong>de</strong> compuetos<br />
farmaceúticos reguladores <strong>de</strong> la vía <strong>de</strong> Hedgehog. Solicitud <strong>de</strong><br />
Patente <strong>de</strong> Invención 200602497 (30 sep 2006). Oficina Española<br />
<strong>de</strong> Patentes y marcas (CSIC-Ministerio <strong>de</strong> Educación y Ciencia).<br />
Organizador <strong>de</strong> congresos<br />
Meeting organizer<br />
ELSO meeting, Molecular Biology in Development. September 1-4<br />
(2005), Dres<strong>de</strong>n, Germany. EMBO conference in Hedgehog-Gli<br />
signaling in <strong>de</strong>velopment and stem cells. September 30.<br />
October 4 (2006), Rome, Italy.<br />
CBM 2005/2006<br />
26<br />
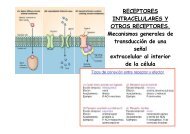
Figura 1. Ptc como receptor <strong>de</strong> Lipoproteínas. La expresión temporal (14<br />
horas) <strong>de</strong> Patched, el receptor <strong>de</strong> Hh, en el compartimento dorsal <strong>de</strong>l disco<br />
<strong>de</strong> ala induce la internalización <strong>de</strong> Lipophorinas (Lp) (apolipoproteinas <strong>de</strong><br />
<strong>Drosophila</strong>). Se pue<strong>de</strong> observar una co-localización en vesículas<br />
endocíticas <strong>de</strong> Ptc, Hh y Lp.<br />
Figure 1. Ptc as a LDLR. Transient ectopic expression (14 hours) of<br />
Patched, the Hh receptor, in the wing imaginal disc induces the<br />
internalization of Lipophorins (Lp) (the <strong>Drosophila</strong> apolipoproteins). Ptc, Hh<br />
and Lp are found in the same endocytic vesicles.<br />
Figura 2. Mo<strong>de</strong>lo <strong>de</strong> formación <strong>de</strong>l gradiente morfogenético <strong>de</strong> Hedgehog.Para la formación <strong>de</strong>l gradiente morfogenético <strong>de</strong> Hh, Hh con sus<br />
modificaciones lipídicas (Hh-Np) se empaqueta como partícula lipoproteica e interacciona con los componentes <strong>de</strong> la matriz extracelular,<br />
Shifed/DmWIF y los Heparan sulfato proteoglycanos (HSPG). Hh sin sus modificaciones lipídicas (Hh-N) al no empaquetarse como partícula<br />
lipoproteica y no interaccionar con los componentes <strong>de</strong> la matriz extracelular forma un gradiente mucho mas extendido. Tanto en mutantes para<br />
Shf como para los HSPG, en los que se pier<strong>de</strong> la proteína Shf, Hh no pue<strong>de</strong> difundir.<br />
Figure 2. Diagram of Hh gradient formation. Lipid-modified Hh (Hh-Np) is packaged as a lipoprotein particle to spread through the extracellular<br />
matrix. For a correct spreading, Hh interacts with the extracellular matrix components, Shifted/DmWIF and the HSPG. Hh without lipid<br />
modifications (Hh-N) shows an exten<strong>de</strong>d gradient because Hh is not appropriated packaged as a lipoprotein particle and does not interact with<br />
the extracellular matrix components. Hh spreading is blocked either in shf or in HSPG mutants, in which Shf/DmWIF protein is lost.<br />
27