Drosophila - Severo Ochoa - Universidad Autónoma de Madrid
Drosophila - Severo Ochoa - Universidad Autónoma de Madrid
Drosophila - Severo Ochoa - Universidad Autónoma de Madrid
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Jefe <strong>de</strong> línea /<br />
Group Lea<strong>de</strong>r:<br />
Francisco J. Moreno<br />
Agregación <strong>de</strong> tau y su implicación<br />
en la enfermedad <strong>de</strong> Alzheimer<br />
Assembly of tau protein and its<br />
implications in Alzheimer´s disease<br />
B4<br />
Publicaciones<br />
Publications<br />
Resumen <strong>de</strong> investigación<br />
Research summary<br />
Santa-María, I., Smith, M.A., Perry, G., Hernán<strong>de</strong>z, F., Avila, J.,<br />
Moreno, F.J. (2005). Effect of quinones on microtubule polymerization:<br />
a link between oxidative stress and cytoskeletal alterations in<br />
Alzheimer's disease. Biochim. Biophys. Acta 1740, 472-80.<br />
Personal Científico /<br />
Scientific Staff:<br />
Juan S. Jiménez<br />
María José Benítez<br />
Concepción Pérez<br />
Becarios Predoctorales /<br />
Predoctoral Fellows:<br />
Ismael Santa-María<br />
Alejandro Barrantes<br />
Biología Celular Cell Biology<br />
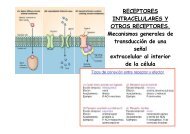
Las tauopatías son alteraciones neurológicas que tienen un<br />
factor común, la presencia <strong>de</strong> agregados aberrantes <strong>de</strong> tau<br />
fosforilada (una proteína asociada a microtúbulos). Los<br />
filamentos helicoidales apareados (PHFs) obtenidos a partir<br />
<strong>de</strong> cerebros <strong>de</strong> enfermos <strong>de</strong> Alzheimer (AD) están formados<br />
esencialmente por moléculas <strong>de</strong> tau hiperfosforiladas. En la<br />
AD, tau interacciona con menor afinidad con los<br />
microtúbulos y esto hace que se autoagregue formando<br />
estructuras aberrantes. Probablemente este proceso se ve<br />
favorecido por la presencia <strong>de</strong> otras moléculas. Establecer<br />
cuales son las zonas <strong>de</strong> tau que participan en el proceso <strong>de</strong><br />
agregación y que facilitan la formación <strong>de</strong> filamentos y las<br />
estructuras moleculares implicadas en la agregación es<br />
esencial para compren<strong>de</strong>r cuales son los mecanismos <strong>de</strong> la<br />
agregación patológica <strong>de</strong> tau.<br />
Después <strong>de</strong> establecerse que tau es el principal<br />
componente <strong>de</strong> los PHFs, se han realizado numerosos<br />
intentos para obtener y caracterizar in vitro, a los PHFs. Por<br />
tanto, se han investigado “nuevas condiciones” que<br />
favorezcan la agregación <strong>de</strong> tau. Nuestros resultados<br />
establecen una conexión mecanicista directa entre<br />
fosforlilación y agregación, a través <strong>de</strong> la formación <strong>de</strong><br />
estructuras en α-hélice. Hemos investigado la facilidad con<br />
que se agregan diferentes fragmentos y variantes <strong>de</strong> tau en<br />
presencia <strong>de</strong> reconocidos agentes inductores que agregan<br />
a tau y nos ha permitido mapearla y establecer que es la<br />
tercera repetición, con la cuál interacciona con los<br />
microtúbulos, la secuencia mínima necesaria para que tau<br />
se agregue, formando filamentos. A<strong>de</strong>más, mediante una<br />
novedosa técnica inmunofluorescente, nos ha permitido<br />
estudiar y caracterizar las estructuras <strong>de</strong> los PHFs,<br />
proce<strong>de</strong>ntes <strong>de</strong> los cerebros <strong>de</strong> los AD o los agregados<br />
obtenidos in vitro, mediante la utilización <strong>de</strong> anticuerpos<br />
especificos <strong>de</strong> tau o con tioflavina (ThS). A<strong>de</strong>más, hemos<br />
iniciado estudios sobre la interacción <strong>de</strong> tau con DNA y<br />
otras proteínas implicadas en neuro<strong>de</strong>generación mediante<br />
resonancia superficial <strong>de</strong> plasmón.<br />
También, hemos <strong>de</strong>mostrado utilizando células intactas que<br />
productos <strong>de</strong>rivados <strong>de</strong> la oxidación <strong>de</strong> la dopamina (la<br />
dopamina quinona que es neurotóxica), un neurotransmisor<br />
implicado en la enfermedad <strong>de</strong> Parkinson o una serie <strong>de</strong><br />
<strong>de</strong>rivados quinónicos, análogos <strong>de</strong>l coenzima Q,<br />
promueven la polimerización <strong>de</strong> la tubulina y tau,<br />
respectivamente. Por último, nuestros resultados sugieren<br />
una conexión entre el daño oxidativo y el establecimiento <strong>de</strong><br />
las tauopatías.<br />
Tauopathies are neurological disor<strong>de</strong>rs with a common<br />
feature, the presence of aberrant phosphotau aggregates.<br />
Paired helical filaments (PHFs) isolated from patients with<br />
Alzheimer’s disease (AD) mainly consist of the microtubuleassociated<br />
protein tau in a hyperphosphorylated form. In<br />
AD, tau binds with lower affinity to microtubules and it selfaggregates<br />
into aberrant structures. Probably helped by<br />
other molecules. Knowing what regions of the protein tau are<br />
involved in its aggregation into aberrant filaments and what<br />
molecular structure is induced by aggregation are critical<br />
steps towards un<strong>de</strong>rstanding the mechanisms involved in<br />
the pathological aggregation of tau.<br />
After the discovery that tau protein was the main component<br />
of PHFs, several attempts were ma<strong>de</strong> to obtain and to<br />
charaterize PHFs in vitro. Consequently, “new conditions”<br />
that could facilitate tau aggregation have been investigated.<br />
Our resuls provi<strong>de</strong> a direct mechanistic connection between<br />
phosphorylation and polymerization in tau, by which<br />
appears to involve formation of α-helix structure. We<br />
investigated the propensity to form fibrillar0 aggregates of a<br />
variety of fragments and variants of the tau protein un<strong>de</strong>r the<br />
influence of different tau fibrillization inducers and we have<br />
mapped the in vitro fibrillization hotspot of tau onto the third<br />
repeat of its microtubule binding domain. Additionally, by<br />
using a novel immunofluorescence method, we have<br />
studied and characterized thereaction of isolated tau<br />
filaments from the brain of AD patients, or in vitro assembled<br />
polymers with specific antibodies, or with thioflavins (ThS).<br />
Additionally, we have initiated the study of tau protein<br />
interactions with DNA and other proteins involved in<br />
neuro<strong>de</strong>generation by Surface Plasmon Resonance.<br />
We have also <strong>de</strong>monstrated in intact cells that oxidized<br />
products of dopamine (neurotoxic dopamine quinone), a<br />
neurotransmitter involved in Parkinson’s disease and<br />
Coenzyme analogs, promote tau and tubulin polymerization,<br />
respectively. Our results support a link between oxidative<br />
damage and the onset of tauopathies.<br />
Santa-María, I., Hernán<strong>de</strong>z, F., Smith, M.A., Perry, G., Avila, J., Moreno,<br />
F.J. (2005). Neurotoxic dopamine quinone facilitates the assembly of tau<br />
into fibrillar polymers. Mol. Cell. Biochem. 278, 203-12.<br />
Martinez, A., Alonso, M., Castro, A., Dorronsoro, I., Gelpi, J.L., Luque,<br />
F.J., Perez, C. and Moreno, F.J. (2005). SAR and 3D-QSAR studies on<br />
thiadíazolidinone <strong>de</strong>rivatives: exploration of structural requirements for<br />
glycogen synthase kinase 3 inhibitors. J. Med. Chem. 48, 7103-7112.<br />
Mendieta, J., Fuertes, M.A., Kunjishapatham, R., Santa-María, I.,<br />
Moreno, F.J., Alonso, C., Gago, F., Munoz, V., Avila, J. and Hernán<strong>de</strong>z,<br />
F. (2005). Phosphorylation modulates the alpha-helical structure and<br />
polymerization of a pepti<strong>de</strong> from the third tau microtubule-binding<br />
repeat. Biochim. Biophys. Acta 1721, 16-26.<br />
Santa-María, I., Perez, M., Hernán<strong>de</strong>z, F., Muñoz, V., Moreno, F.J. and<br />
Avila, J. (2006) In vitro tau fibrillization: mapping protein regions.<br />
Biochim. Biophys. Acta 1762, 683-92.<br />
Barrantes, A., Navarro, P.J., Benítez, M.J. and Jiménez, J.S. (2006). A<br />
DNA and histone immobilization method to study DNA-histone<br />
interactions by surface plasmon resonance.<br />
Analytic. Biochem. 352, 151-153.<br />
Santa-María, I., Perez, M., Hernán<strong>de</strong>z, F., Avila, J., Moreno, F.J. (2006).<br />
Characteristics of the binding of thioflavin S to tau paired helical<br />
filaments. J. Alzh. Dis. 9, 279-85.<br />
Avila, J., Santa-María, I., Pérez, M., Hernán<strong>de</strong>z, F. and Moreno, F.J.<br />
(2006). Tau phosphorylation, aggregation, and cell toxicity.<br />
J. Biomed. Biotechnol. 1-5.<br />
Figura 1. Efecto <strong>de</strong> las quinonas en cultivos primarios <strong>de</strong> neuronas <strong>de</strong> hipocampo.<br />
Figure 1. Effect of quinones on cultured hippocampal neurons.<br />
CBM 2005/2006<br />
46<br />
Figura 2. Mo<strong>de</strong>lo <strong>de</strong> la estructura <strong>de</strong> un PHF. Se sugiere la presencia <strong>de</strong> una región central estable,<br />
que se tiñe preferentemente por la ThS, y dos extremos dinámicos.<br />
Figure 2. Mo<strong>de</strong>l of PHF structure. It is suggested the presence of a central stable region,<br />
that is preferentially stained with ThS; and two dynamic ends.<br />
47