Water and Solute Permeability of Plant Cuticles: Measurement and ...
Water and Solute Permeability of Plant Cuticles: Measurement and ...
Water and Solute Permeability of Plant Cuticles: Measurement and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
246 8 Effects <strong>of</strong> Temperature on Sorption <strong>and</strong> Diffusion <strong>of</strong> <strong>Solute</strong>s <strong>and</strong> Penetration <strong>of</strong> <strong>Water</strong><br />
log k ∗<br />
infinite<br />
20<br />
15<br />
10<br />
5<br />
0<br />
- 5<br />
log k ∗<br />
infinite = - 3.27 + (1.44E D 10 -3 /R)<br />
MX<br />
-10<br />
0 2 4 6 8 10 12 14 16 18<br />
CM<br />
log k ∗<br />
infinite = - 5.24 +(1.37E D 10 -3 /R)<br />
Hedera helix/IAA<br />
Enthalpy <strong>of</strong> diffusion x 10 -3 x R -1<br />
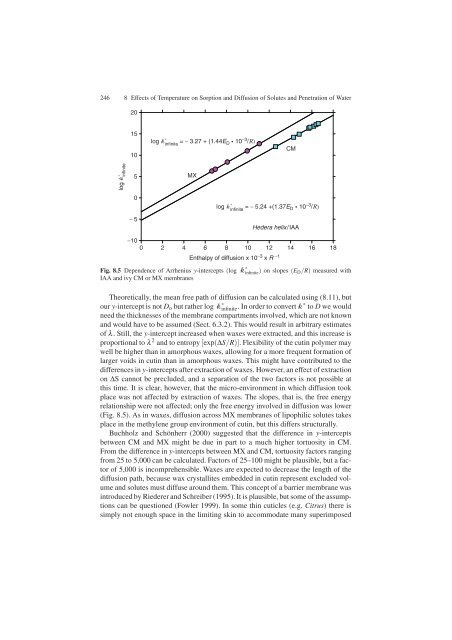
Fig. 8.5 Dependence <strong>of</strong> Arrhenius y-intercepts (log k ∗<br />
infinite ) on slopes (ED/R) measured with<br />
IAA <strong>and</strong> ivy CM or MX membranes<br />
Theoretically, the mean free path <strong>of</strong> diffusion can be calculated using (8.11), but<br />
our y-intercept is not Do but rather log k ∗ infinite. In order to convert k ∗ to D we would<br />
need the thicknesses <strong>of</strong> the membrane compartments involved, which are not known<br />
<strong>and</strong> would have to be assumed (Sect. 6.3.2). This would result in arbitrary estimates<br />
<strong>of</strong> λ . Still, the y-intercept increased when waxes were extracted, <strong>and</strong> this increase is<br />
proportional to λ 2 <strong>and</strong> to entropy [exp(∆S/R)]. Flexibility <strong>of</strong> the cutin polymer may<br />
well be higher than in amorphous waxes, allowing for a more frequent formation <strong>of</strong><br />
larger voids in cutin than in amorphous waxes. This might have contributed to the<br />
differences in y-intercepts after extraction <strong>of</strong> waxes. However, an effect <strong>of</strong> extraction<br />
on ∆S cannot be precluded, <strong>and</strong> a separation <strong>of</strong> the two factors is not possible at<br />
this time. It is clear, however, that the micro-environment in which diffusion took<br />
place was not affected by extraction <strong>of</strong> waxes. The slopes, that is, the free energy<br />
relationship were not affected; only the free energy involved in diffusion was lower<br />
(Fig. 8.5). As in waxes, diffusion across MX membranes <strong>of</strong> lipophilic solutes takes<br />
place in the methylene group environment <strong>of</strong> cutin, but this differs structurally.<br />
Buchholz <strong>and</strong> Schönherr (2000) suggested that the difference in y-intercepts<br />
between CM <strong>and</strong> MX might be due in part to a much higher tortuosity in CM.<br />
From the difference in y-intercepts between MX <strong>and</strong> CM, tortuosity factors ranging<br />
from 25 to 5,000 can be calculated. Factors <strong>of</strong> 25–100 might be plausible, but a factor<br />
<strong>of</strong> 5,000 is incomprehensible. Waxes are expected to decrease the length <strong>of</strong> the<br />
diffusion path, because wax crystallites embedded in cutin represent excluded volume<br />
<strong>and</strong> solutes must diffuse around them. This concept <strong>of</strong> a barrier membrane was<br />
introduced by Riederer <strong>and</strong> Schreiber (1995). It is plausible, but some <strong>of</strong> the assumptions<br />
can be questioned (Fowler 1999). In some thin cuticles (e.g. Citrus) there is<br />
simply not enough space in the limiting skin to accommodate many superimposed