the Molten Salt Energy Technologies Web Site
the Molten Salt Energy Technologies Web Site
the Molten Salt Energy Technologies Web Site
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ANP PROJECT QUARTERLY PROGRESS REPORT<br />
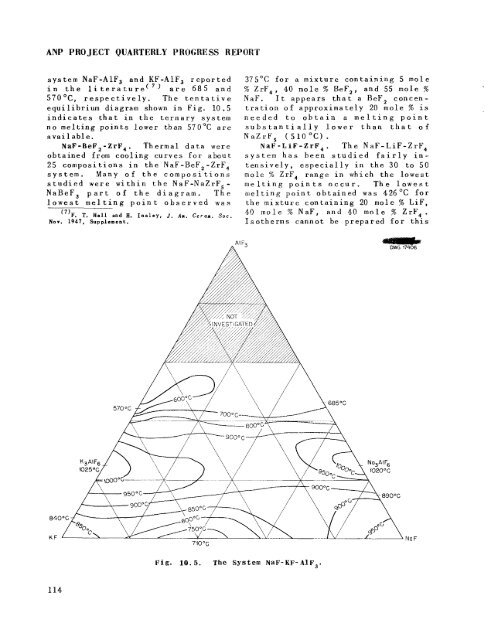
sys tem NaF-A1F3 and KF-AlF, reported<br />
in <strong>the</strong> literature(') are 685 and<br />
570°C, respectively. The tentative<br />
equilibrium diagram shown in Fig. 10.5<br />
indicates that in <strong>the</strong> ternary system<br />
no melting points lower than 570°C are<br />
avai 1 ab1 e.<br />
NaF-BeF,-ZrF,. Thermal data were<br />
obtained from cooling curves for about<br />
25 compositions in <strong>the</strong> NaF-SeF,-ZrF,<br />
system. Many of <strong>the</strong> compositions<br />
studied were within <strong>the</strong> NaF-NaZrF5 -<br />
NaBeF3 part of <strong>the</strong> diagram. The<br />
lowest melting point observed was<br />
(7)F. T. Hall and H. Insley, J. Am. Cera.. SOC.<br />
Nor. 1947, Supplement.<br />
114<br />
Fig. 10.5. The System NaF-KF- AlF,.<br />
375°C: for a mixture containing 5 mole<br />
% ZrF,, 40 mole % BeF2, and 55 mole %<br />
NaF. It appears that a ReF, concen-<br />
tration of approximately 20 mole % is<br />
needed to obtain a melting point<br />
substantially lower than that of<br />
NaZrF5 (510 "C) .<br />
NaF-LiF-ZrF,. The NaF-LiF-ZrF,<br />
system has been studied fairly in-<br />
tensively, especially in <strong>the</strong> 30 to 50<br />
mole % ZrF4 range in which <strong>the</strong> lowest<br />
melting points occur. The lowest<br />
melting point obtained was 426OC for<br />
<strong>the</strong> mixture containing 20 mole % LiF,<br />
40 mole % NaF, and 40 mole % ZrF,.<br />
Iso<strong>the</strong>rms cannot be prepared for this<br />
890°C


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)












