the Molten Salt Energy Technologies Web Site
the Molten Salt Energy Technologies Web Site
the Molten Salt Energy Technologies Web Site
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ANP PROJECT QUARTERLY PROGRESS REPORT<br />
-<br />
0- 4<br />
B- 5<br />
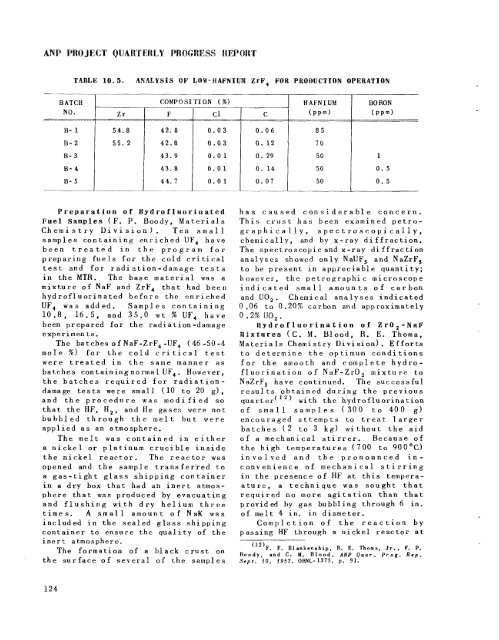
TABLE 10.5. ANALYSIS OF LOW- APNIUM ZrF, FOR PRODUCTION OPERATION<br />
RATCH<br />
~. COMPO SI TI ON ( %)<br />
.. .. . .<br />
HAFNIUM<br />
NO. Zr F Cl C<br />
43. a 0.01<br />
44. I 0.01<br />
P rep ar at i o n o f My d 1-0 f 1 u o r i PI a& e d<br />
Fuel samples (F. P. Boody, Materials<br />
Chemistry Division). Ten small<br />
samples containing enriched UF, have<br />
been treated in <strong>the</strong> program for<br />
preparing fuels for <strong>the</strong> cold critical<br />
test and for radiation-damage tests<br />
in <strong>the</strong> MTR. The base material was a<br />
mixture of NaF and ZrF, that had been<br />
hydrofluorinated before <strong>the</strong> cnriched<br />
UF, was added. Samples containing<br />
10.8, 16.5, and 35.0 w t % UF, have<br />
been prepared for <strong>the</strong> radiation-damage<br />
experiments.<br />
The batches of NaF-ZrF, -IJF, (45 -50 -4<br />
mole %) for <strong>the</strong> cold critical test<br />
were treated in <strong>the</strong> same manner as<br />
bat,ches containingnormal UF,. However,<br />
<strong>the</strong> batches required for radiationdamage<br />
tests were small (10 to 20 g),<br />
and <strong>the</strong> procedure was modified so<br />
that <strong>the</strong> HF, H,, and He gases were not<br />
bubbled through <strong>the</strong> melt but were<br />
applied as an atmosphere.<br />
The melt was contained in ei<strong>the</strong>r<br />
a nickel or platinum crucible inside<br />
<strong>the</strong> nickel reactor. The reactor wzs<br />
opened and <strong>the</strong> sample transfer'red to<br />
a gas-tight glass shipping cont,ainer<br />
in a dry box that had an inert atmoswhere<br />
that wits produced by evacuating<br />
and flushirig with dry helium three<br />
times. A small amount of NaK was<br />
included in <strong>the</strong> sealed glass shipping<br />
container to ensure <strong>the</strong> quality of <strong>the</strong><br />
inert atmosphere.<br />
The formation of a black crust on<br />
<strong>the</strong> surface of several of <strong>the</strong> samples<br />
124<br />
1 0.07<br />
...~ .... I ...... ~<br />
BO RON<br />
1 1 0. 14 50 0.5<br />
0.5<br />
50<br />
I<br />
I<br />
has caused considerable concern.<br />
This crust has been examined petrogr<br />
a p hi c a l l y , s p e c t r o s c o p i c a l l y ,<br />
chemically, and by x-ray diffraction.<br />
The spectroscopic and x-ray diffraction<br />
analyses showed only NaUF5 and NaZrFs<br />
to be present in appreciable quantity;<br />
however, <strong>the</strong> petrographic microscope<br />
indicat,ed s m a l l amounts of carbon<br />
and UO,. Chemical analyses indicated<br />
0.06 to 0.20% carbon and approximately<br />
0 .2% [JQ,.<br />
Hydroflusrination of ZrO,-N*aF<br />
k!lxtvres (C. M. Blood, R. E. Thoma,<br />
Materials Chemistry Division). Efforts<br />
to determine <strong>the</strong> optimum conditions<br />
for <strong>the</strong> smooth and complete hydrofluorination<br />
of NaF-ZrQ, mixtur~ to<br />
NaZrF5 have continued. The successful<br />
results obtained during <strong>the</strong> previous<br />
quarter' 2, with <strong>the</strong> hydrofluorination<br />
of small samples (300 to 400 g)<br />
encouraged attempts to treat 1 arger<br />
batches (2 to 3 kg) without <strong>the</strong> aid<br />
of a mechanical stirrer. Because of<br />
<strong>the</strong> high temperatures (700 to 900°C)<br />
involved and <strong>the</strong> pronounced inconvenience<br />
of mechanical stirring<br />
in <strong>the</strong> presence of HF at this temperaature,<br />
a technique was sought that<br />
required no more agitation than that<br />
provided by gas bubbling through 6 in.<br />
of melt 4 in. in diameter.<br />
Completion of <strong>the</strong> reaction by<br />
passing HF through a nickel reactor at<br />
(12)F. F. Blankenship, R. E. Thomn, Jr., F. P.<br />
Boody, and C. M. Blood, ANP Quar. Prog. Rep.<br />
Sept. 10, 1952. OWL-1375, p. 91.


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)












