the Molten Salt Energy Technologies Web Site
the Molten Salt Energy Technologies Web Site
the Molten Salt Energy Technologies Web Site
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ANP lJHO JECT QUARTERLY PHOGRESS REPORT<br />
-. ~ .- ........<br />
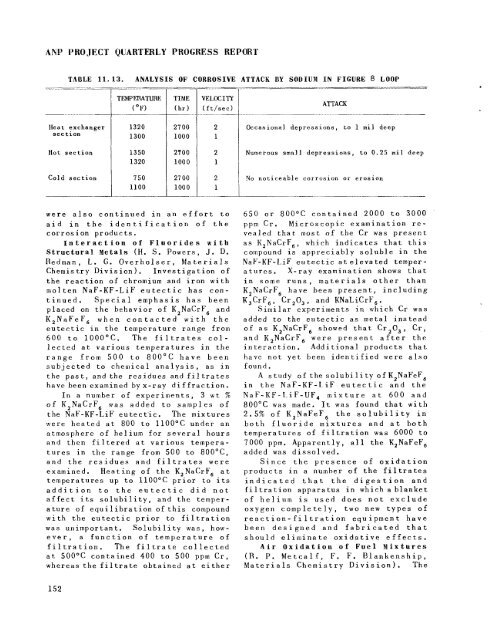
TABLE 11.13. ANALYSIS OF CORROSIVE ATTACK BY SODIUM IN FIGURE 8 LQOP<br />
.<br />
~ __ ___.<br />
....... . __ ......... ....... ~ .____ .-..-.<br />
- .......... ____<br />
Heat exchanger<br />
section<br />
Hot section<br />
Cold section<br />
TEMPFmTURE<br />
(OF)<br />
1320<br />
1300<br />
1350<br />
1320<br />
750<br />
1100<br />
TIME<br />
(hr)<br />
2700<br />
1000<br />
2'100<br />
1000<br />
27 00<br />
1000<br />
were also continued in an effort to<br />
aid in <strong>the</strong> identification of <strong>the</strong><br />
corrosion products.<br />
Interaction of Fluorides urith<br />
Structural Netals (H. S. Powers, J. D.<br />
Redman, L. G. Overholser, Materials<br />
Chemistry Division). Investigation of<br />
<strong>the</strong> reaction of chromium and iron with<br />
molten NaF-KF-LiF eutectic has con-<br />
tinued. Special emphasis has been<br />
placed on <strong>the</strong> behavior of K2NaCrF6 and<br />
K2NaFeF6 when contacted with <strong>the</strong><br />
eutectic in <strong>the</strong> temperature range from<br />
600 to 1000°C. The filtrates col-<br />
lected at various temperatures in <strong>the</strong><br />
range from 500 to 800°C have been<br />
subjected to chemical analysis, as in<br />
<strong>the</strong> past, and <strong>the</strong> residues and filtrates<br />
have been examined by x-ray diffraction.<br />
In a number of experiments, 3 w t %<br />
of K2NaCrF6 was added to samples of<br />
<strong>the</strong> NaF-KF-LiF eutectic. The mixtures<br />
were heated at 800 to 1100°C under an<br />
atmosphere of helium for several hours<br />
and <strong>the</strong>n filtered at various teinpera-<br />
tures in <strong>the</strong> range from 500 to 800"C,<br />
and <strong>the</strong> residues and filtrates were<br />
examined. Heating of <strong>the</strong> K2NaCrF6 at<br />
temperatures up to 1100°C prior to its<br />
addition to <strong>the</strong> eutectic did not<br />
affect its solubility, and <strong>the</strong> temper-<br />
ature of equilibration of this compound<br />
with <strong>the</strong> eutectic prior to filtration<br />
was unimportant. Solubility was, how-<br />
ever, a function of temperature of<br />
filtration. The filtrate collected<br />
at 500°C contained 400 to 500 ppm Cr,<br />
whereas <strong>the</strong> filtrate obtained at ei<strong>the</strong>r<br />
152<br />
...<br />
2<br />
1<br />
2<br />
1<br />
2<br />
1<br />
ATTACK<br />
Occasional depressions, to 1 m il deep<br />
. -<br />
Numerous small depressions, to 0.25 mil deep<br />
No noticeable corrosion or erosion<br />
650 or 800°C contained 2000 to 3000<br />
ppm Cr. Microscopic examination re-<br />
vealed that most of <strong>the</strong> Cr was present<br />
as K2NaCrF6, which indicates that this<br />
compound is appreciably soliible in <strong>the</strong><br />
NaF-KF-LiF eutectic at elevated temper-<br />
atures. X-ray examination shows that<br />
in some runs, materials o<strong>the</strong>r than<br />
KzNaCrF6 have been present, including<br />
K,CrF6, Cr203, and KNaLiCrF,.<br />
Similar experiments in which Cr was<br />
added to <strong>the</strong> eutectic as metal instead<br />
of as K2NaCrFs showed that Cr20J, Cr,<br />
and K,NaCrF, were present after <strong>the</strong><br />
i n t e r a c t i on. Add i t ion a 1 p rod u c t s t ha t<br />
have not yet been identified were also<br />
found.<br />
A study of <strong>the</strong> solubility of K,NaFeF6<br />
in <strong>the</strong> NaF-KF-1iF eutectic and <strong>the</strong><br />
NaF-KF-LiF-UF,, mixture at 600 and<br />
800°C was made. It was found that with<br />
2.5% of K2NaFeF6 <strong>the</strong> solubility in<br />
both fluoride mixtures and at both<br />
temperatures of filtration was 6000 to<br />
7000 ppm. Apparently, all <strong>the</strong> K,NaFeF,<br />
added was dissolved.<br />
Since <strong>the</strong> presence of oxidation<br />
products in a number of <strong>the</strong> filtrates<br />
indicated that <strong>the</strong> digestion and<br />
filtration apparatus in which a blanket<br />
of helium is used does not exclude<br />
oxygen completely, two new types of<br />
reaction- fi ltration equipment have<br />
been designed and fabricated that<br />
should eliminate oxidative effects.<br />
Air Oxidation of Fuel Mixtures<br />
(R. P. Metcalf, F. F. Blankenship,<br />
Materials Chemistry Division). The


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)












