the Molten Salt Energy Technologies Web Site
the Molten Salt Energy Technologies Web Site
the Molten Salt Energy Technologies Web Site
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
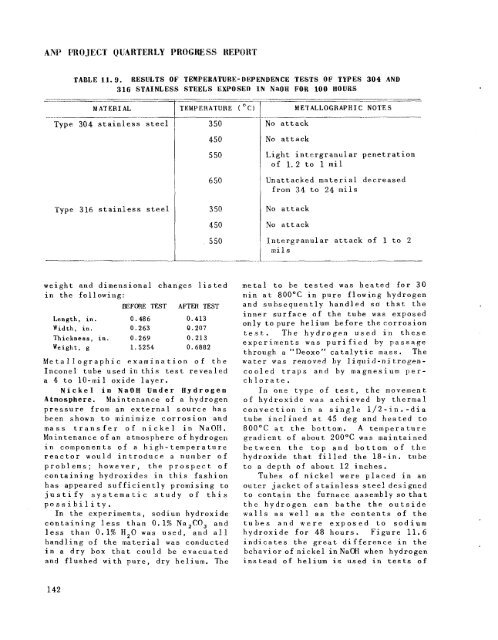
ANP PROJECT QUARTERLY PRQGIXSS HEPORT<br />
TABLE 11.9. RESULTS OF TEmPERATURE-DERENDENCE TESTS OF TYPES 304 AND<br />
316 STAINLESS STEELS EXPOSED IN NaOH FOR 100 HOURS<br />
_.___ ...... ~ ^._... _____ _-- ___ ......... -<br />
.- ~ ____ _ _ _ _ ____<br />
MATERIAL<br />
TEMPERATURE ( OC)<br />
METALLOGRAPHIC NOTES<br />
. ._ _._.<br />
Type 304 stainless steel<br />
Type 316 stainless steel<br />
- .....--- ~-<br />
3 50<br />
450<br />
5 50<br />
6 50<br />
3 50<br />
4 50<br />
5 50<br />
weight and dimensional changes listed<br />
in <strong>the</strong> following:<br />
BEFORE TEST AFTER TEST<br />
Length, in. 0.486 0.413<br />
Width, in. 0.263 Q. 207<br />
Thickness, in. 0.269 0.213<br />
Weight, g 1.5254 0.6882<br />
Metallographic examination of <strong>the</strong><br />
Inconel tube used in this test revealed<br />
a 4 to 10-mil oxide layer.<br />
Nickel in NaOH Under Hydrogen<br />
Atmosphere. Maintenance of a hydrogen<br />
pressure from an external source has<br />
been shown to minimize corrosion and<br />
mass transfer of nickel in NaO11.<br />
Maintenance of an atmosphere of hydrogen<br />
in components of a high-temperature<br />
reactor would introduce a number of<br />
problems; however, <strong>the</strong> prospect of<br />
containing hydroxides in this fashion<br />
has appeared sufficiently promising to<br />
justify systematic study of this<br />
possi bil i t y.<br />
In <strong>the</strong> experiments, sodium hydroxide<br />
containing less than 0.1% Na,CO, and<br />
less than 0.1% H,O was used, and all<br />
handling of <strong>the</strong> material was conducted<br />
in a dry box that could be evacuated<br />
and flushed with pure, dry helium. The<br />
142<br />
No attack<br />
No attack<br />
_.__.__.._I<br />
Light intergranular penetration<br />
of 1.2 to 1 m il<br />
Unattacked material decreased<br />
from 34 to 24 m i l s<br />
No attack<br />
No attack<br />
Intergranular attack of 1 t o 2<br />
mi 1 s<br />
metal to be tested was heated for 30<br />
min at 800°C in pure flowing hydrogen<br />
and subsequently handled so that <strong>the</strong><br />
inner surface of <strong>the</strong> tube was exposed<br />
only to pure helium before <strong>the</strong> corrosion<br />
test. The hydrogen used in <strong>the</strong>se<br />
experiments was purified by passage<br />
through a "Deoxo" catalytic mass. The<br />
water was removed by liquid-nitrogen-<br />
cooled traps and by magnesium per-<br />
chlorate.<br />
In one type of test, <strong>the</strong> movement<br />
of hydroxide was achieved by <strong>the</strong>rmal<br />
convection in a single 1/2-in.-dia<br />
tube inclined at 45 deg and heated to<br />
800°C at <strong>the</strong> bottom. A temperature<br />
gradient of about 200°C was maintained<br />
between <strong>the</strong> top and bottom of <strong>the</strong><br />
hydroxide that filled <strong>the</strong> 18-in. tube<br />
to a depth of about 12 inches.<br />
Tubes of nickel were placed in an<br />
outer jacket of stainless steel designed<br />
to contain <strong>the</strong> furnace assembly so that<br />
<strong>the</strong> hydrogen can ba<strong>the</strong> <strong>the</strong> outside<br />
walls as well as <strong>the</strong> contents of <strong>the</strong><br />
tubes and were exposed to sodium<br />
hydroxide for 48 hours. Figure 11.6<br />
indicates <strong>the</strong> great difference in <strong>the</strong><br />
behavior of nickel inNaOH when hydrogen<br />
instead of helium is used in tests of


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)












