ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
AT= 136OOF<br />
OPERATING TIME = 312 hr<br />
MAXIMUM CORROSION<br />
PENETRATION = 0 001 in.<br />
i6O0F<br />
NOTE EQUILIBRIUM LOOP SURFACE TEMPERATURES<br />
ARE GIVEN.<br />
-<br />
0°F<br />
4651<br />
BOILER AND<br />
VAPOR<br />
SEPARATOR<br />
SECTION<br />
30°F<br />
-<br />
1 1 2 3 4 5 1<br />
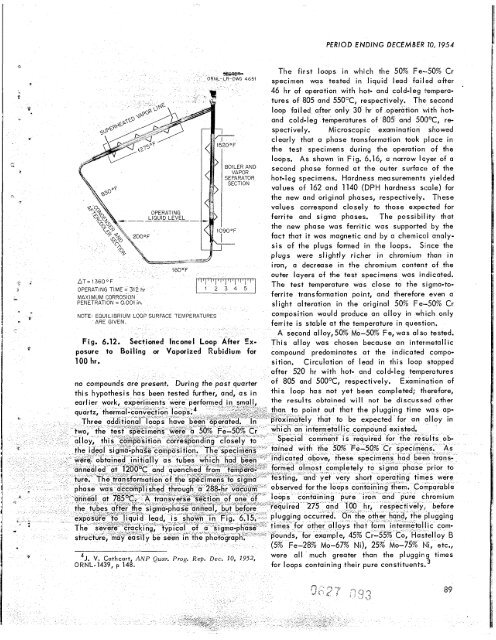
Fig. 6.12. Sectioned lnconel Loop After Ex-<br />
posure to Boiling or Vaporized Rubidium for<br />
100 hr.<br />
During - <strong>the</strong> past quarter<br />
this hypo<strong>the</strong>sis has been tested fur<strong>the</strong>r, and, as in<br />
,.<br />
PERlOD ENDING DECEMBER 70, 1954<br />
The first loops in which <strong>the</strong> 50% Fe--50% Cr<br />
specimen was tested in liquid lead failed after<br />
46 hr of operation with hot- and cold-leg Vempera-<br />
tures of 805 and 550°C, respectively. The second<br />
loop failed after only 30 hr of o<br />
and cold-leg temperatures of 805 and 5OO0C, re-<br />
spect ivel y. Microscopic examination showed<br />
clearly that a phase transformation took piece in<br />
<strong>the</strong> test specimens during <strong>the</strong> operation of <strong>the</strong><br />
loops. As shown in Fig, 6.16, a narrow layer of a<br />
second phase formed at <strong>the</strong> outer surface of <strong>the</strong><br />
hot-leg specimens, Hardness measurements yielded<br />
values of 162 and 1140 (DPH hardness scale) for<br />
<strong>the</strong> new and original phases, respectively. These<br />
values correspond closely to those expected for<br />
ferrite and sigma phases, The possibility that<br />
<strong>the</strong> new phase was ferritic was supported by <strong>the</strong><br />
fact that it was magnetic and by a chemicctl analy-<br />
sis of <strong>the</strong> plugs formed in <strong>the</strong> loops. Siince <strong>the</strong><br />
plugs were slightly richer in chromium than in<br />
iron, a decrease in <strong>the</strong> chromium content of <strong>the</strong><br />
outer layers of <strong>the</strong> test specimens was indicated.<br />
The test temperature was close to <strong>the</strong> sigma-fo-<br />
ferrite transformation point, and <strong>the</strong>refore even a<br />
slight alteration in <strong>the</strong> original 50% Fe--50% Cr<br />
composition would produce an alloy in which only<br />
ferrite is stable at <strong>the</strong> temperature in question.<br />
A second alloy, 50% Mo-50% Fe, was a1 so tested.<br />
This alloy was chosen because an intermetallic<br />
compound predominates at <strong>the</strong> indicated compo-<br />
sition, Circulation of lead in this loop stopped<br />
after 520 hr with hot- and cold-leg temperatures<br />
of 805 and 5OO0C, respectively. Examination of<br />
this loop has not yet been completed; <strong>the</strong>refore,<br />
<strong>the</strong> results obtained will not be discussed o<strong>the</strong>r<br />
for loops containing <strong>the</strong>ir pure constituents.<br />
'


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)












