ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ANP QUARTERLY PROGRESS REPORT<br />
0.2<br />
0.1<br />
0.02<br />
‘1 .AMINAR FLOW<br />
RP<br />
SOLID POINTS INDICATE CURRENT FLOW IN FLUID<br />
LENGTH (in.)<br />
199<br />
UNCLASSIFIED<br />
<strong>ORNL</strong>-LR-QWG 4453<br />
2 5 I 04 2 5 {05<br />
Re, REYNOLDS NUMBER<br />
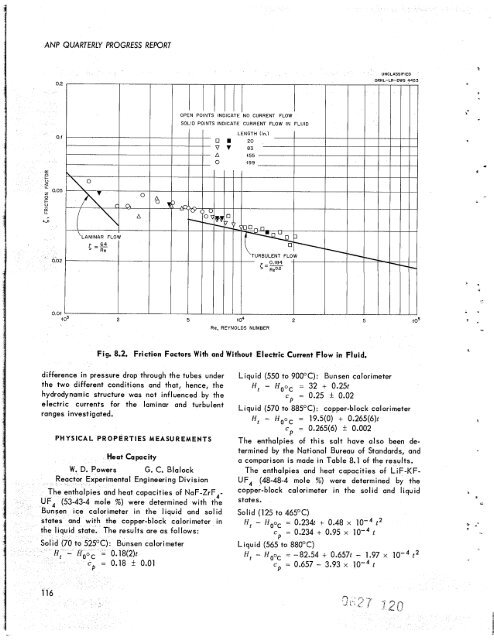
Fig. 8.2. Friction Factors With and Without Electric Current Flow in Fluid.<br />
difference in pressure drop through <strong>the</strong> tubes under<br />
<strong>the</strong> two different conditions and that, hence, <strong>the</strong><br />
hydrodynamic structure was not influenced by <strong>the</strong><br />
electric currents for <strong>the</strong> laminar and turbulent<br />
ranges investigated.<br />
PH YSlCA L PR 0 PERT IES M EASU REM E NTS<br />
Heat Capacity<br />
W. D. Powers G. C. Blalock<br />
rimenta I Engineering Division<br />
and heat capacities of NaF-ZrF4-<br />
le %) were determined with <strong>the</strong><br />
rimeter in <strong>the</strong> liquid and solid<br />
tes and with <strong>the</strong> copper-block calorimeter in<br />
he results are as follows:<br />
Solid (70 to 525OC): Bunsen calorimeter<br />
c = 0.18 5 0.01<br />
P<br />
Liquid (550 to 9OOOC): Bunsen calorimeter<br />
H, - HOoC = 32 + 0.25t<br />
c = 0.25 k 0.02<br />
P<br />
Liquid (570 to 885OC): copper-block calorimeter<br />
H, - HOoC = 19.5(0) + 0.265(6)t<br />
c = 0.265(6) t 0.002<br />
P<br />
The enthalpies of this salt have also been de-<br />
termined by <strong>the</strong> National Bureau of Stendards, and<br />
a comparison is made in Table 8.1 of <strong>the</strong> results.<br />
The enthalpies and heat capacities of LiF-KF-<br />
UF, (48-48-4 mole 5%) were determined by <strong>the</strong><br />
copper-block calorimeter in <strong>the</strong> solid and liquid<br />
states.<br />
Solid (125 to 465°C)<br />
H, - HOoC = 0.234t + 0.48 x t2<br />
c = 0.234 + 0.95 x 0-4 t<br />
P<br />
Liquid (565 to 88OOC)<br />
H, - HOoC = -82.54 + 0.657t<br />
c = 0.657 - 3.93 x<br />
P<br />
- 1.97 10-4 t2<br />
0-4 t<br />
.<br />
f<br />
x


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)












