ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ANP QUARTERLY PROGRESS REPORT<br />
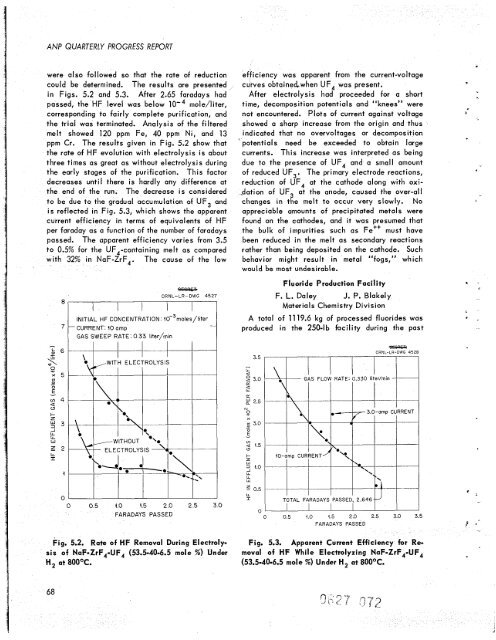
were also followed so that <strong>the</strong> rate of reduction<br />
could be determined. The results are presented<br />
in Figs. 5.2 and 5.3. After 2.65 faradays had<br />
passed, <strong>the</strong> HF level was below lo', mole/liter,<br />
corresponding to fairly complete purification, and<br />
<strong>the</strong> trial was terminated. Analysis of <strong>the</strong> filtered<br />
melt showed 120 ppm Fe, 40 ppm Ni, and 13<br />
ppm Cr. The results given in Fig. 5.2 show that<br />
<strong>the</strong> rate of HF evolution with electrolysis is about<br />
three times as great as without electrolysis during<br />
<strong>the</strong> early stages of <strong>the</strong> purification. This factor<br />
decreases unti I <strong>the</strong>re is hardly any difference at<br />
<strong>the</strong> end of <strong>the</strong> run. The decrease is considered<br />
to be due to <strong>the</strong> gradual accumulation of UF, and<br />
is reflected in Fig. 5.3, which shows <strong>the</strong> apparent<br />
current efficiency in terms of equivalents of HF<br />
per faraday as a function of <strong>the</strong> number of faradays<br />
passed. The apparent efficiency varies from 3.5<br />
to 0.5% for <strong>the</strong> UF4-containing melt as compared<br />
with 32% in NaF-ZrF,. The cause<br />
-<br />
of <strong>the</strong> low<br />
<strong>ORNL</strong>-LR-DWG 4527<br />
1<br />
k 6<br />
5<br />
2<br />
-<br />
x 5<br />
u)<br />
-<br />
m 4<br />
a<br />
(3<br />
c<br />
z<br />
Y 3<br />
_J<br />
LL<br />
LL<br />
W<br />
E 2<br />
LL<br />
I<br />
4<br />
GAS SWEEP RATE: o 33 \iter/min<br />
0<br />
0 0 5 10 15 2.0 25 30<br />
FARADAYS PASSED<br />
Fig. 5.2. Rate of HF Removal During Electroly-<br />
sis of NaF-ZrF,-UF, (53.5-40-6.5 mole X ) Under<br />
H, at 800'C.<br />
68<br />
efficiency was apparent from <strong>the</strong> current-voltage<br />
curves obtainedwhen UF, was present.<br />
After electrolysis had proceeded for a short<br />
time, decomposition potentials and "knees" were<br />
not encountered. Plots of current against voltage<br />
showed a sharp increase from <strong>the</strong> origin and thus<br />
indicated that no overvoltages or decomposition<br />
potentials need be exceeded to obtain large<br />
currents. This increase was interpreted as being<br />
due to <strong>the</strong> presence of UF, and a small amount<br />
of reduced UF,. The primary electrode reactions,<br />
reduction of UF, at <strong>the</strong> cathode along with oxi-<br />
.dation of UF, at <strong>the</strong> anode, caused <strong>the</strong> over-all<br />
changes in <strong>the</strong> melt to occur very slowly. No<br />
appreciable amounts of precipitated metals were<br />
found on <strong>the</strong> cathodes, and it was presumed that<br />
<strong>the</strong> bulk of impurities such as Fett must have<br />
been reduced in <strong>the</strong> melt as secondary reactions<br />
ra<strong>the</strong>r than being deposited on <strong>the</strong> cathode. Such<br />
behavior might result in metal "fogs," which<br />
would be most undesirable.<br />
Fluoride Production Facility ..<br />
F. L. Daley J. P. Blakely<br />
Mater ials Chemistry Division<br />
A total of 1119.6 kg of processed fluorides was<br />
produced in <strong>the</strong> 250-lb facility during <strong>the</strong> past<br />
* ,<br />
I<br />
><br />
3.5<br />
$ 3.0<br />
LT<br />
2<br />
E<br />
2.5<br />
N<br />
0<br />
x<br />
0 3.0<br />
-<br />
0<br />
E<br />
:<br />
a<br />
b-<br />
z<br />
W<br />
;<br />
LL<br />
U<br />
W<br />
U<br />
r<br />
1.5<br />
1.0<br />
0.5<br />
0<br />
TOTAL FARADAYS PASSED, 2.646<br />
esBE&l<br />
<strong>ORNL</strong>-LR-OWG 4528<br />
0 0.5 4.0 4.5 2.0 2.5 3.0 3.5<br />
FARADAYS PASSED f -<br />
Fig. 5.3. Apparent Current Efficiency for Re-<br />
moval of HF While Electrolyzing NaF-ZrF4-UF,<br />
(53.5-40-6.5 mole %) Under H, at 800%<br />
J


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)












