ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site
ORNL-1816 - the Molten Salt Energy Technologies Web Site
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ANP QUARTERLY PROGRESS REPORT<br />
Consequently, experiments were run with mixtures<br />
prepared without <strong>the</strong> preliminary HF treatment, and<br />
<strong>the</strong> resulting batches were studied closely for<br />
such contaminants as oxides, sulfur, iron, chromium,<br />
and nickel. In all cases <strong>the</strong> oxide content<br />
was not detectable, and <strong>the</strong> sulfur content well<br />
below 100 ppm. Comparison with HF-treated<br />
batches showed no detectable differences. The<br />
iron, chromium, and nickel concentrations were,<br />
in general, lower than those resulting from HFtreated<br />
batches. In an attempt to produce <strong>the</strong><br />
NaF-KF-LiF eutectic (1 1.5-42.0-46.5 mole 7%) plus<br />
14 wt % UF, without <strong>the</strong> preliminary HF treatment,<br />
it was found that <strong>the</strong> previous difficulty of inconsistent<br />
results still remained.<br />
A study of <strong>the</strong> reaction<br />
F, --+ 4UF,<br />
ali-metal fluorides has shown<br />
that potassium fluoride has a strong inhibiting<br />
completion of <strong>the</strong> reaction. It was<br />
<strong>the</strong> KF concentration of <strong>the</strong> eutectic<br />
mixture (NaF-KF-LiF, 11.5-42.0-46.5 mole %), a<br />
little better than 50% conversion of <strong>the</strong> UF, to<br />
UF, could be expected. With this in mind, new<br />
attempts were made to produce consistent batches,<br />
and it soon became evident that <strong>the</strong> handling<br />
technique was quite important. After several<br />
changes in processing techniques were tried, <strong>the</strong><br />
most promising method was chosen as a standard<br />
procedure. Briefly, this method utilizes separate<br />
purification steps for <strong>the</strong> main constituents involved.<br />
The eutectic (NaF-KF-LiF, 11.5-42.0-46.5<br />
mole %) is first heated and stripped with H,. The<br />
melt is cooled and <strong>the</strong> UF, is added, after which<br />
<strong>the</strong> melt is again heated and stripped. The melt<br />
is <strong>the</strong>n cooled, and uranium metal is added. The<br />
final heating and stripping are <strong>the</strong>n carried out,<br />
and <strong>the</strong> batch is transferred to a storage can.<br />
In this manner, drying and purification of <strong>the</strong><br />
alkali metal fluorides are accomplished without<br />
danger of hydrolyzing or oxidizing <strong>the</strong> UF,; also,<br />
thorough mixing of <strong>the</strong> UF, is provided before <strong>the</strong><br />
ranium metal is added. A fur<strong>the</strong>r advantage is<br />
that <strong>the</strong> batch is well purified and miscellaneous<br />
side reactions of impurities with <strong>the</strong> uranium metal<br />
are kept to a minimum.<br />
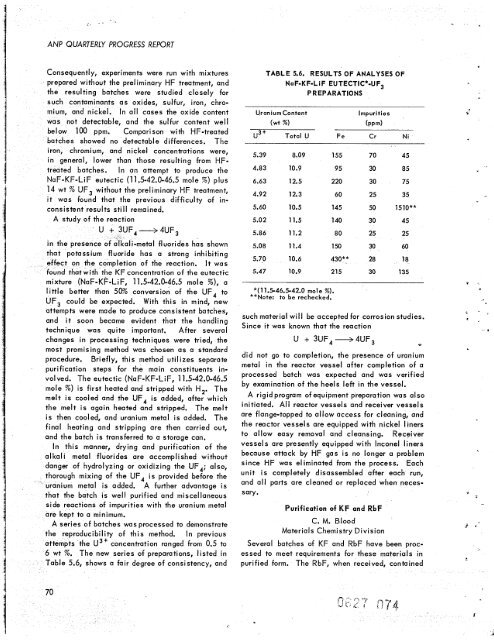
A series of batches was processed to demonstrate<br />
producibility of this method. In previous<br />
ts <strong>the</strong> U3' concentration ranged from 0.5 to<br />
6 wt %. The new series of preparations, listed in<br />
able 5.6, shows a fair degree of consistency, and<br />
TABLE 5.6. RESULTS OF ANALYSES OF<br />
NaF-KF-Li F EUTECTIC*-UF3<br />
PREPARATIONS<br />
Uranium Content lmpuri ties<br />
(wt W) (PPm)<br />
U3' Total U Fe Cr Ni<br />
5.39 8.09 155 70 45<br />
4.83 10.9 95 30 85<br />
6.63 12.5 220 30 75<br />
4.92 12.3 60 25 35<br />
5.60 10.5 145 50 1510**<br />
5.02 11.5 140 30 45<br />
5.86 11.2 80 25 25<br />
5.08 11.4 150 30 60<br />
5.70 10.6 430** 28 18<br />
5.47 10.9 215 30 135<br />
*(11.546.5-42.0 mole W).<br />
**Note: to be rechecked.<br />
such material will be accepted for corrosion studies.<br />
Since it was known that <strong>the</strong> reaction<br />
U + 3UF4+4UF,<br />
did not go to completion, <strong>the</strong> presence of uranium<br />
metal in <strong>the</strong> reactor vessel after completion of a<br />
processed batch was expected and was verified<br />
by examination of <strong>the</strong> heels left in <strong>the</strong> vessel.<br />
A rigid program of equipment preparation was also<br />
initiated. All reactor vessels and receiver vessels<br />
are flange-topped to allow access for cleaning, and<br />
<strong>the</strong> reactor vessels are equipped with nickel liners<br />
to allow easy removal and cleansing. Receiver<br />
vessels are presently equipped with lnconel liners<br />
because attack by HF gas is no longer a problem<br />
since HF was eliminated from <strong>the</strong> process. Each<br />
unit is completely disassembled after each run,<br />
and all parts are cleaned or replaced when neces-<br />
sary. c<br />
Purification of KF and RbF<br />
C. M. Blood<br />
Mater io Is Chemistry D i vi s ion<br />
Several batches of KF and RbF have been proc-<br />
essed to meet requirements for <strong>the</strong>se materials in<br />
purified form. The RbF, when received, contained<br />
0<br />
7 .<br />
t<br />
f .<br />
1<br />
- t<br />
I


![Review of Molten Salt Reactor Physics Calculations [Disc 2]](https://img.yumpu.com/21979492/1/190x247/review-of-molten-salt-reactor-physics-calculations-disc-2.jpg?quality=85)












