The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Results and Discussion<br />
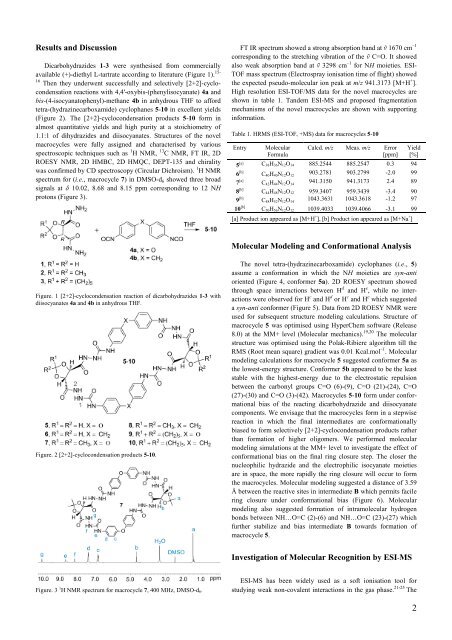
Dicarbohydrazides 1-3 were synthesised from commercially<br />
available (+)-diethyl L-tartrate according to literature (Figure 1). 15-<br />
16 <strong>The</strong>n they underwent successfully and selectively [2+2]-cyclocondensation<br />
reactions with 4,4'-oxybis-(phenylisocyanate) 4a and<br />
bis-(4-isocyanatophenyl)-methane 4b in anhydrous THF to afford<br />
tetra-(hydrazinecarboxamide) cyclophanes 5-10 in excellent yields<br />
(Figure 2). <strong>The</strong> [2+2]-cyclocondensation products 5-10 form in<br />
almost quantitative yields and high purity at a stoichiometry <strong>of</strong><br />
1.1:1 <strong>of</strong> dihydrazides and diisocyanates. Structures <strong>of</strong> the novel<br />
macrocycles were fully assigned and characterised by various<br />
spectroscopic techniques such as 1 H NMR, 13 C NMR, FT IR, 2D<br />
ROESY NMR, 2D HMBC, 2D HMQC, DEPT-135 and chirality<br />
was confirmed by CD spectroscopy (Circular Dichroism). 1 H NMR<br />
spectrum for (i.e., macrocycle 7) in DMSO-d 6 showed three broad<br />
signals at δ 10.02, 8.68 and 8.15 ppm corresponding to 12 NH<br />
protons (Figure 3).<br />
FT IR spectrum showed a strong absorption band at ṽ 1670 cm –1<br />
corresponding to the stretching vibration <strong>of</strong> the ṽ C=O. It showed<br />
also weak absorption band at ṽ 3298 cm –1 for NH moieties. ESI-<br />
TOF mass spectrum (Electrospray ionisation time <strong>of</strong> flight) showed<br />
the expected pseudo-molecular ion peak at m/z 941.3173 [M+H + ].<br />
High resolution ESI-TOF/MS data for the novel macrocycles are<br />
shown in table 1. Tandem ESI-MS and proposed fragmentation<br />
mechanisms <strong>of</strong> the novel macrocycles are shown with supporting<br />
information.<br />
Table 1. HRMS (ESI-TOF, +MS) data for macrocycles 5-10<br />
Entry<br />
Molecular<br />
Formula<br />
Calcd. m/z Meas. m/z Error<br />
[ppm]<br />
Yield<br />
[%]<br />
5 [a] C 38H 36N 12O 14 885.2544 885.2547 0.3 94<br />
6 [b] C 40H 40N 12O 12<br />
903.2781 903.2799 -2.0 99<br />
7 [a] C 42H 44N 12O 14<br />
941.3150 941.3173 2.4 89<br />
8 [b] C 44H 48N 12O 12 959.3407 959.3439 -3.4 90<br />
9 [b] C 48H 52N 12O 14<br />
1043.3631 1043.3618 -1.2 97<br />
10 [b] C 50H 56N 12O 12 1039.4033 1039.4066 -3.1 99<br />
[a] Product ion appeared as [M+H + ], [b] Product ion appeared as [M+Na + ]<br />
Molecular Modeling and Conformational Analysis<br />
Figure. 1 [2+2]-cyclocondensation reaction <strong>of</strong> dicarbohydrazides 1-3 with<br />
diisocyanates 4a and 4b in anhydrous THF.<br />
Figure. 2 [2+2]-cyclocondensation products 5-10.<br />
<strong>The</strong> novel tetra-(hydrazinecarboxamide) cyclophanes (i.e., 5)<br />
assume a conformation in which the NH moieties are syn-anti<br />
oriented (Figure 4, conformer 5a). 2D ROESY spectrum showed<br />
through space interactions between H d and H e , while no interactions<br />
were observed for H c and H d or H c and H e which suggested<br />
a syn-anti conformer (Figure 5). Data from 2D ROESY NMR were<br />
used for subsequent structure modeling calculations. Structure <strong>of</strong><br />
macrocycle 5 was optimised using HyperChem s<strong>of</strong>tware (Release<br />
8.0) at the MM+ level (Molecular mechanics). 19,20 <strong>The</strong> molecular<br />
structure was optimised using the Polak-Ribiere algorithm till the<br />
RMS (Root mean square) gradient was 0.01 Kcal.mol –1 . Molecular<br />
modeling calculations for macrocycle 5 suggested conformer 5a as<br />
the lowest-energy structure. Conformer 5b appeared to be the least<br />
stable with the highest-energy due to the electrostatic repulsion<br />
between the carbonyl groups C=O (6)-(9), C=O (21)-(24), C=O<br />
(27)-(30) and C=O (3)-(42). Macrocycles 5-10 form under conformational<br />
bias <strong>of</strong> the reacting dicarbohydrazide and diisocyanate<br />
components. We envisage that the macrocycles form in a stepwise<br />
reaction in which the final intermediates are conformationally<br />
biased to form selectively [2+2]-cyclocondensation products rather<br />
than formation <strong>of</strong> higher oligomers. We performed molecular<br />
modeling simulations at the MM+ level to investigate the effect <strong>of</strong><br />
conformational bias on the final ring closure step. <strong>The</strong> closer the<br />
nucleophilic hydrazide and the electrophilic isocyanate moieties<br />
are in space, the more rapidly the ring closure will occur to form<br />
the macrocycles. Molecular modeling suggested a distance <strong>of</strong> 3.59<br />
Å between the reactive sites in intermediate B which permits facile<br />
ring closure under conformational bias (Figure 6). Molecular<br />
modeling also suggested formation <strong>of</strong> intramolecular hydrogen<br />
bonds between NH…O=C (2)-(6) and NH…O=C (23)-(27) which<br />
further stabilize and bias intermediate B towards formation <strong>of</strong><br />
macrocycle 5.<br />
Investigation <strong>of</strong> Molecular Recognition by ESI-MS<br />
Figure. 3 1 H NMR spectrum for macrocycle 7, 400 MHz, DMSO-d 6.<br />
ESI-MS has been widely used as a s<strong>of</strong>t ionisation tool for<br />
studying weak non-covalent interactions in the gas phase. 21-25 <strong>The</strong><br />
2