- Page 1 and 2: The Development of Novel Antibiotic
- Page 3 and 4: Declaration of Authorship I, Hany N
- Page 5 and 6: Abstract Over the past few decades,
- Page 7 and 8: Chapter 2 Trianglimine Chemistry
- Page 9 and 10: Figure 2.4 Trianglimines 10-12 with
- Page 11 and 12: Abbreviations ACN AcOH Ala Acetonit
- Page 13 and 14: RMS ROESY SjGST TFA THF TLC TMS TOC
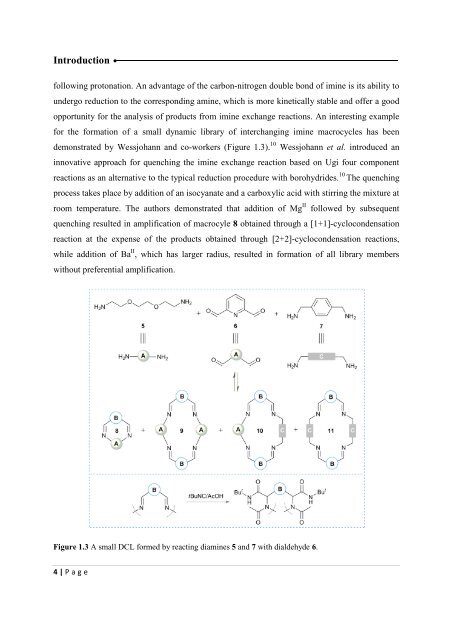
- Page 15: Introduction complete remodel of th
- Page 19 and 20: Introduction Lehn and co-workers re
- Page 21 and 22: Introduction Figure 1.8 Generation
- Page 23 and 24: Introduction Figure 1.11 Synthetic
- Page 25 and 26: Introduction Figure 1.13 Generation
- Page 27 and 28: Introduction 1.3.11 Boronic ester e
- Page 29 and 30: Introduction References 1. E. Mouli
- Page 31 and 32: Introduction 51. B. Shi, M. F. Grea
- Page 33 and 34: Introduction Figure 2.2 shows compu
- Page 35 and 36: Introduction incorporate β-cyclode
- Page 37 and 38: Introduction Trianglamine-Zn(R) 2 c
- Page 39 and 40: Introduction 24. J. Gajewy, J. Gawr
- Page 41 and 42: Scope of Work Scope of Work In this
- Page 43 and 44: Scope of Work can be obtained from
- Page 45 and 46: Scope of Work Figure 3.4 Structures
- Page 47 and 48: Scope of Work References 1. G. Wrig
- Page 49 and 50: Organic & Biomolecular Chemistry Ci
- Page 51 and 52: Downloaded by Jacobs University Bre
- Page 53 and 54: View Online Downloaded by Jacobs Un
- Page 55 and 56: View Online Downloaded by Jacobs Un
- Page 57 and 58: View Online Downloaded by Jacobs Un
- Page 59 and 60: View Online Downloaded by Jacobs Un
- Page 61 and 62: View Online Downloaded by Jacobs Un
- Page 63 and 64: Paper 2 Rapid Commun. Mass Spectrom
- Page 65 and 66: Trianglimine formation mechanism by
- Page 67 and 68:
Trianglimine formation mechanism by
- Page 69 and 70:
Trianglimine formation mechanism by
- Page 71 and 72:
Trianglimine formation mechanism by
- Page 73 and 74:
Trianglimine formation mechanism by
- Page 75 and 76:
Paper 3 Org. Biomol. Chem., 2012, 1
- Page 77 and 78:
View Online Downloaded by Jacobs Un
- Page 79 and 80:
View Online Downloaded by Jacobs Un
- Page 81 and 82:
View Online Table 2 Distances (Å)
- Page 83 and 84:
View Online Downloaded by Jacobs Un
- Page 85 and 86:
Paper 4 Chem. Eur. J., 2012, submit
- Page 87 and 88:
(4R,5R)- and (4S,5S)-dimethyl-2,2-d
- Page 89 and 90:
that two distinct conformational is
- Page 91 and 92:
the thimble was recharged with fres
- Page 93 and 94:
Paper 5 Rapid Commu. Mass Spectrom.
- Page 95 and 96:
INTRODUCTION No single class of dru
- Page 97 and 98:
EXPERIMENTAL All the solvents and r
- Page 99 and 100:
(b) Refluxing macrocycles 5, 6 and
- Page 101 and 102:
Scheme 1. Graphical representation
- Page 103 and 104:
Figure 1. A plot of relative intens
- Page 105 and 106:
In contrast, formation of library m
- Page 107 and 108:
Figure 5. Generated dynamic library
- Page 109 and 110:
macrocycle 32 from the dynamic libr
- Page 111 and 112:
The polar carbamate group participa
- Page 113 and 114:
[2] WHO World health report 2003. h
- Page 115 and 116:
[32] B. Lienard, N. Selevsek, N. Ol
- Page 117 and 118:
FULL PAPER Synthesis and ESI-MS Com
- Page 119 and 120:
ability of the technique to provide
- Page 121 and 122:
aimed at assessing molecular recogn
- Page 123 and 124:
Paper 7 manuscript Synthesis, self-
- Page 125 and 126:
2 Similar to the case of trianglimi
- Page 127 and 128:
4 Receptor 7 showed enhanced recogn
- Page 129 and 130:
6 The newly synthesized receptors s
- Page 131 and 132:
8 27. Nour, H. F.; Hourani, N.; Kuh
- Page 133 and 134:
120| P a g e Appendix
- Page 135 and 136:
Supplementary Information: Suppleme
- Page 137 and 138:
Supplementary Material (ESI) for Or
- Page 139 and 140:
Supplementary Material (ESI) for Or
- Page 141 and 142:
Supplementary Material (ESI) for Or
- Page 143 and 144:
Supplementary Material (ESI) for Or
- Page 145 and 146:
Supplementary Material (ESI) for Or
- Page 147 and 148:
Supplementary Material (ESI) for Or
- Page 149 and 150:
Supplementary Material (ESI) for Or
- Page 151 and 152:
Supplementary Material (ESI) for Or
- Page 153 and 154:
Monitoring the [3+3]-cyclocondensat
- Page 155 and 156:
Monitoring the cyclocondensation re
- Page 157 and 158:
Product ions appeared as [M+H] + Fi
- Page 159 and 160:
Scheme 1. Assigned higher oligomers
- Page 161 and 162:
Supplementary Information: Synthesi
- Page 163 and 164:
Scheme 4. Proposed fragmentation me
- Page 165 and 166:
DMSO Figure 1. 1 H-NMR spectrum for
- Page 167 and 168:
Figure 6. 13 C-NMR spectrum for (4S
- Page 169 and 170:
Intens. 7 x10 1.5 200.7 1.0 0.5 0.0
- Page 171 and 172:
Intens. 7 x10 200.7 1.5 1.0 0.5 0.0
- Page 173 and 174:
Intens. 4 x10 577.2 2.0 1.5 1.0 451
- Page 175 and 176:
HN N O O R R NH O O N N HN R O O O
- Page 177 and 178:
Intens. 5 x10 727.2 2.0 1.5 1.0 O O
- Page 179 and 180:
Intens. 5 x10 697.1 1.5 1.0 728.2 0
- Page 181 and 182:
O O O O HN N O O NH N HN N O O NH N
- Page 183 and 184:
Figure 38. 1 H-NMR spectrum for mac
- Page 185 and 186:
Intens. 5000 276.8 4000 3000 2000 1
- Page 187 and 188:
Intens. 7 x10 599.1 1.5 1.0 0.5 0.0
- Page 189 and 190:
(a) HN N O O NH O O N (b) HN N O NH
- Page 191 and 192:
Scheme 11. Proposed fragmentation m
- Page 193 and 194:
Figure 56. 1 H-NMR spectrum for mac
- Page 195 and 196:
Scheme 14. Proposed fragmentation m
- Page 197 and 198:
Intens. 4 x10 550.9 3 2 1 0 500 100
- Page 199 and 200:
Figure 68. 2D-ROESY spectrum for ma
- Page 201 and 202:
Supplementary Information: Novel sy
- Page 203 and 204:
Intens. 6 x10 0.8 0.6 0.4 240.9 459
- Page 205 and 206:
Figure 9. 1 H-NMR spectrum for macr
- Page 207 and 208:
Figure 15. 1 H-NMR spectrum for mac
- Page 209 and 210:
Intens. 5 x10 1.5 968.1 1.0 0.5 813
- Page 211 and 212:
Intens. 5 x10 897.4 4 2 335.3 707.3
- Page 213 and 214:
Scheme 3. Suggested fragmentation m
- Page 215 and 216:
Figure 35. 1 H-NMR spectrum for mac
- Page 217 and 218:
Molecular modelling data for the co
- Page 219 and 220:
Figure 1. 1 H NMR spectrum for dica
- Page 221 and 222:
Intens. 2000 1493.6091 Host-Guest 1
- Page 223 and 224:
Intens. [%] 100 775.3 80 388.1 60 4
- Page 225 and 226:
Intens. 600 N HN O O 467.1916 400 O
- Page 227 and 228:
Intens. 6000 5000 HN N O O O O NH N
- Page 229 and 230:
Table 1. High resolution ESI-TOF da
- Page 231 and 232:
i Figure 1. 1 H NMR spectrum for ma
- Page 233 and 234:
i Figure 5. 1 H NMR spectrum for ma
- Page 235 and 236:
i j Figure 9. 1 H NMR spectrum for
- Page 237 and 238:
i Figure 13. DEPT-135 spectrum for
- Page 239 and 240:
Scheme 1. General mechanism of frag
- Page 241 and 242:
Intens. [%] 100 80 60 40 20 0 O H N
- Page 243 and 244:
Intens. [%] 100 1131.3 80 60 40 20
- Page 245 and 246:
Intens. 1500 1528.6088 1000 500 150
- Page 247 and 248:
Intens. [%] 100 80 60 40 Free guest
- Page 249 and 250:
Intens. 4 x10 1085.3575 1.25 1.00 0
- Page 251 and 252:
Intens. [%] 100 80 Free guest 586.1
- Page 253 and 254:
Synthesis, self-assembly and ESI-MS
- Page 255 and 256:
O O HN NH HN O O NH HN O 11 O NH O
- Page 257 and 258:
O O HN NH HN O O NH HN O 9 O NH O O
- Page 259 and 260:
H 2 O DMSO DMSO Figure 6. 1 H-NMR a
- Page 261 and 262:
Intens. 5 x10 5 -MS 487.1588 4 3 2
- Page 263 and 264:
Intens. -MS 6 x10 0.8 0.6 0.4 0.2 1
- Page 265 and 266:
Intens. -MS 6 x10 O O 350.9 1.5 1.0
- Page 267 and 268:
Intens. [%] 100 -MS 486.9 Exact str
- Page 269 and 270:
Intens. [%] -MS 100 486.9 80 60 975
- Page 271 and 272:
Intens. [%] +MS 100 80 517.2 Exact
- Page 273 and 274:
Intens. [%] 100 80 60 +MS 388.1 775
- Page 275 and 276:
Intens. [%] 100 +MS 517.2 Exact str
- Page 277 and 278:
Intens. [%] 100 80 +MS 1031.4939 7
- Page 279 and 280:
Intens. [%] 100 +MS 517.2 Exact str
- Page 281 and 282:
Intens. [%] 100 -MS 2 586.1 80 60 4
- Page 283 and 284:
Intens. [%] 100 -MS 2 875.2 Exact s
- Page 285 and 286:
Table 1. High resolution ESI-TOF/MS
- Page 287 and 288:
274 | P a g e
- Page 289 and 290:
276 | P a g e
- Page 291:
4. H. Nour, A. López-Periago, N. K