The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Trianglimine formation mechanism by ESI-TOF MS<br />
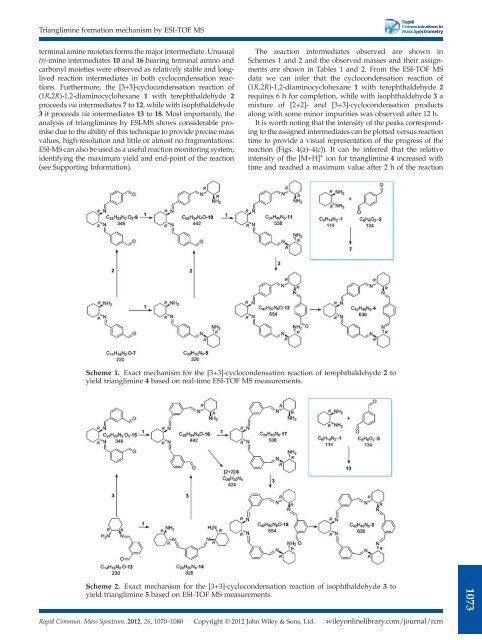
terminal amine moieties forms the major intermediate. Unusual<br />
tri-imine intermediates 10 and 16 bearing terminal amino and<br />
carbonyl moieties were observed as relatively stable and longlived<br />
reaction intermediates in both cyclocondensation reactions.<br />
Furthermore, the [3+3]-cyclocondensation reaction <strong>of</strong><br />
(1R,2R)-1,2-diaminocyclohexane 1 with terephthaldehyde 2<br />
proceeds via intermediates 7 to 12, while with isophthaldehyde<br />
3 it proceeds via intermediates 13 to 18. Most importantly, the<br />
analysis <strong>of</strong> trianglimines by ESI-MS shows considerable promise<br />
due to the ability <strong>of</strong> this technique to provide precise mass<br />
values, high-resolution and little or almost no fragmentations.<br />
ESI-MS can also be used as a useful reaction monitoring system,<br />
identifying the maximum yield and end-point <strong>of</strong> the reaction<br />
(see Supporting Information).<br />
<strong>The</strong> reaction intermediates observed are shown in<br />
Schemes 1 and 2 and the observed masses and their assignments<br />
are shown in Tables 1 and 2. From the ESI-TOF MS<br />
data we can infer that the cyclocondensation reaction <strong>of</strong><br />
(1R,2R)-1,2-diaminocyclohexane 1 with terephthaldehyde 2<br />
requires 6 h for completion, while with isophthaldehyde 3 a<br />
mixture <strong>of</strong> [2+2]- and [3+3]-cyclocondensation products<br />
along with some minor impurities was observed after 12 h.<br />
It is worth noting that the intensity <strong>of</strong> the peaks corresponding<br />
to the assigned intermediates can be plotted versus reaction<br />
time to provide a visual representation <strong>of</strong> the progress <strong>of</strong> the<br />
reaction (Figs. 4(a)–4(c)). It can be inferred that the relative<br />
intensity <strong>of</strong> the [M+H] + ion for trianglimine 4 increased with<br />
time and reached a maximum value after 2 h <strong>of</strong> the reaction<br />
Scheme 1. Exact mechanism for the [3+3]-cyclocondensation reaction <strong>of</strong> terephthaldehyde 2 to<br />
yield trianglimine 4 based on real-time ESI-TOF MS measurements.<br />
Scheme 2. Exact mechanism for the [3+3]-cyclocondensation reaction <strong>of</strong> isophthaldehyde 3 to<br />
yield trianglimine 5 based on ESI-TOF MS measurements.<br />
1073<br />
Rapid Commun. Mass Spectrom. 2012, 26, 1070–1080<br />
Copyright © 2012 John Wiley & Sons, Ltd.<br />
wileyonlinelibrary.com/journal/rcm