The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
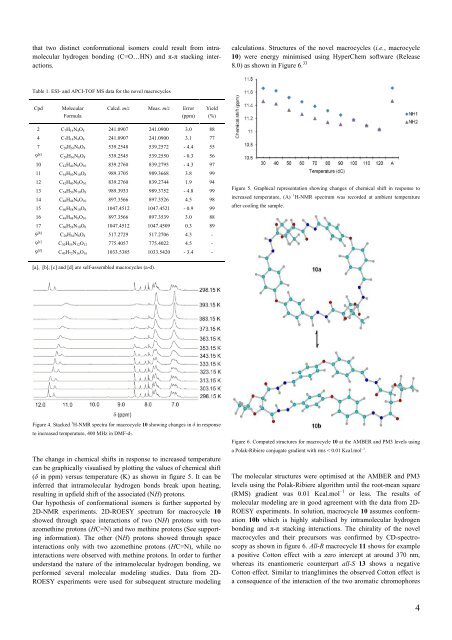
that two distinct conformational isomers could result from intramolecular<br />
hydrogen bonding (C=O…HN) and π-π stacking interactions.<br />
calculations. Structures <strong>of</strong> the novel macrocycles (i.e., macrocycle<br />
10) were energy minimised using HyperChem s<strong>of</strong>tware (Release<br />
8.0) as shown in Figure 6. 33<br />
Table 1. ESI- and APCI-TOF MS data for the novel macrocycles<br />
Cpd<br />
Molecular<br />
Formula<br />
Calcd. m/z Meas. m/z Error<br />
(ppm)<br />
Yield<br />
(%)<br />
2 C 7H 14N 4O 4 241.0907 241.0900 3.0 88<br />
4 C 7H 14N 4O 4 241.0907 241.0900 3.1 77<br />
7 C 20H 36N 8O 8 539.2548 539.2572 - 4.4 55<br />
9 [a] C 20H 36N 8O 8 539.2545 539.2550 - 0.3 56<br />
10 C 42H 40N 8O 10 839.2760 839.2795 - 4.3 97<br />
11 C 54H 50N 10O 8 989.3705 989.3668 3.8 99<br />
12 C 42H 40N 8O 10 839.2760 839.2744 1.9 94<br />
13 C 54H 50N 10O 8 989.3933 989.3752 - 4.8 99<br />
14 C 48H 48N 8O 10 897.3566 897.3526 4.5 98<br />
15 C 60H 58N 10O 8 1047.4512 1047.4521 - 0.9 99<br />
16 C 48H 48N 8O 10 897.3566 897.3539 3.0 88<br />
17 C 60H 58N 10O 8 1047.4512 1047.4509 0.3 89<br />
9 [b] C 20H 36N 8O 8 517.2729 517.2706 4.3 -<br />
9 [c] C 30H 54N 12O 12 775.4057 775.4022 4.5 -<br />
9 [d] C 40H 72N 16O 16 1033.5385 1033.5420 - 3.4 -<br />
Figure 5. Graphical representation showing changes <strong>of</strong> chemical shift in response to<br />
increased temperature, (A) 1 H-NMR spectrum was recorded at ambient temperature<br />
after cooling the sample.<br />
[a], [b], [c] and [d] are self-assembled macrocycles (a-d).<br />
Figure 4. Stacked 1 H-NMR spectra for macrocycle 10 showing changes in δ in response<br />
to increased temperature, 400 MHz in DMF-d 7.<br />
<strong>The</strong> change in chemical shifts in response to increased temperature<br />
can be graphically visualised by plotting the values <strong>of</strong> chemical shift<br />
(δ in ppm) versus temperature (K) as shown in figure 5. It can be<br />
inferred that intramolecular hydrogen bonds break upon heating,<br />
resulting in upfield shift <strong>of</strong> the associated (NH) protons.<br />
Our hypothesis <strong>of</strong> conformational isomers is further supported by<br />
2D-NMR experiments. 2D-ROESY spectrum for macrocycle 10<br />
showed through space interactions <strong>of</strong> two (NH) protons with two<br />
azomethine protons (HC=N) and two methine protons (See supporting<br />
information). <strong>The</strong> other (NH) protons showed through space<br />
interactions only with two azomethine protons (HC=N), while no<br />
interactions were observed with methine protons. In order to further<br />
understand the nature <strong>of</strong> the intramolecular hydrogen bonding, we<br />
performed several molecular modeling studies. Data from 2D-<br />
ROESY experiments were used for subsequent structure modeling<br />
Figure 6. Computed structures for macrocycle 10 at the AMBER and PM3 levels using<br />
a Polak-Ribiere conjugate gradient with rms ˂ 0.01 Kcal.mol –1 .<br />
<strong>The</strong> molecular structures were optimised at the AMBER and PM3<br />
levels using the Polak-Ribiere algorithm until the root-mean square<br />
(RMS) gradient was 0.01 Kcal.mol –1 or less. <strong>The</strong> results <strong>of</strong><br />
molecular modeling are in good agreement with the data from 2D-<br />
ROESY experiments. In solution, macrocycle 10 assumes conformation<br />
10b which is highly stabilised by intramolecular hydrogen<br />
bonding and π-π stacking interactions. <strong>The</strong> chirality <strong>of</strong> the novel<br />
macrocycles and their precursors was confirmed by CD-spectroscopy<br />
as shown in figure 6. All-R macrocycle 11 shows for example<br />
a positive Cotton effect with a zero intercept at around 370 nm,<br />
whereas its enantiomeric counterpart all-S 13 shows a negative<br />
Cotton effect. Similar to trianglimines the observed Cotton effect is<br />
a consequence <strong>of</strong> the interaction <strong>of</strong> the two aromatic chromophores<br />
4