The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
H. F. Nour, A. M. Lopez-Periago and N. Kuhnert<br />
4, 5 and 6. <strong>The</strong> closer the nucleophilic amino and the electrophilic<br />
carbonyl groups are in space the more rapidly ring<br />
closure will occur. <strong>The</strong> lowest energy conformations for intermediates<br />
10, 12, 16 and 18 are shown in Fig. 5. It can be<br />
concluded from the computational calculations that the<br />
nucleophilic and electrophilic groups in intermediates 12<br />
and 18 are located 4.62 and 4.25 Å apart in space. This<br />
certainly allows facile ring closure due to conformational bias.<br />
Furthermore, the formation <strong>of</strong> the [2+2]-cyclocondensation<br />
macrocycle 6 is in complete agreement with the computed<br />
structure 16 in which the reactive sites are 4.66 Å separated.<br />
Unlike the case with intermediate 16, intermediate 10 cannot<br />
cyclocondense to the corresponding [2+2]-macrocycle because,<br />
in terephthaldehyde, the dihedral angle O=CC=O biases<br />
intermediate 10 to a conformation in which the reactive<br />
nucleophilic and electrophilic sites are separated by 9.74 Å. This<br />
distance does not permit cyclocondensation to the [2+2]-<br />
macrocycle but it rather permits further condensation with<br />
one molecule <strong>of</strong> (1R,2R)-1,2-diaminocyclohexane 1 and another<br />
molecule <strong>of</strong> terephthaldehyde 2 to form trianglimine 4.<br />
Similar to the [3+3]-cyclocondensation reactions <strong>of</strong> (1R,2R)-<br />
1,2-diaminocyclohexane 1 with tere- 2 and isophthaldehyde 3<br />
which constitute symmetrical dialdehyde building blocks, the<br />
cyclocondensation reaction <strong>of</strong> (1R,2R)-1,2-diaminocyclohexane<br />
1 with non-symmetrical dialdehydes was investigated. <strong>The</strong><br />
reaction was monitored as described with tere- 2 and isophthaldehyde<br />
3 using ESI-TOF MS. Interestingly, a total <strong>of</strong> ten different<br />
intermediates were detected and their structures were<br />
assigned based on their high-resolution m/z values. To the best<br />
<strong>of</strong> our knowledge, the detection and structural assignment <strong>of</strong><br />
ten reaction intermediates in a one single reaction have not<br />
previously been reported, illustrating the power <strong>of</strong> mass spectrometry<br />
in mechanistic studies <strong>of</strong> multistep and cascade<br />
reactions. Recently, we reported the synthesis <strong>of</strong> 3-phenoxybiphenyl-4,4′-dicarbaldehyde<br />
19 from the corresponding<br />
4-bromo-2-fluorobenzaldehyde via nucleophilic aromatic<br />
substitution reaction with phenol followed by Suzuki coupling<br />
with 4-formylphenylboronic acid. [18] It is worth noting that<br />
3-phenoxybiphenyl-4,4′-dicarbaldehyde 19 constitutes a nonsymmetrical<br />
dialdehyde building block and its reaction with<br />
(1R,2R)-1,2-diaminocyclohexane 1 has resulted in the formation<br />
<strong>of</strong> a mixture <strong>of</strong> regioisomeric C 3 -symmetrical 30 and nonsymmetrical<br />
31 trianglimines as shown in Fig. 6. [18]<br />
<strong>The</strong> mechanism <strong>of</strong> the [3+3]-cyclocondensation reaction<br />
was studied and confirmed by ESI-TOF MS (Scheme 3).<br />
A solution <strong>of</strong> 3-phenoxybiphenyl-4,4′-dicarbaldehyde 19<br />
in DCM was stirred at room temperature with (1R,2R)-<br />
1,2-diaminocyclohexane 1 at 0.1 M for 8 h. Aliquots were<br />
taken every 2 h, diluted with DCM and a few drops <strong>of</strong><br />
MeOH were added before it was directly infused into the<br />
ESI-TOF mass spectrometer. Intermediates 20–29 were<br />
detected and assigned based on their high-resolution m/z<br />
values (Table 3). Figure 7 shows the ESI-TOF mass spectra<br />
for all the intermediates which were detected 2 h after the<br />
start<strong>of</strong>thereaction.<br />
Dynamic reversibility <strong>of</strong> trianglimine formation<br />
Should the cyclocondensation reaction be fully reversible as<br />
suggested by the reversible nature <strong>of</strong> the imine bond (C=N) formation,<br />
the design <strong>of</strong> dynamic combinatorial libraries would be<br />
feasible. [33] Ultimate pro<strong>of</strong> <strong>of</strong> reversibility has been defined by<br />
Sanders and co-workers. [34–43] Here reversibility is demonstrated<br />
by reaching the same equilibrium composition <strong>of</strong> a mixture<br />
from different starting points <strong>of</strong> the reaction system. For<br />
this reason we undertook a series <strong>of</strong> crossover experiments. In<br />
a first series we mixed two sets <strong>of</strong> the non-symmetrical trisubstituted<br />
trianglimines 31 and 32 in DCM and the reaction was<br />
monitored with ESI-TOF MS (Scheme 4).<br />
Trianglimines 31 and 32 were synthesized following<br />
our strategy from non-symmetrical dialdehyde building<br />
blocks. [18] <strong>The</strong> two macrocycles were mixed together in DCM<br />
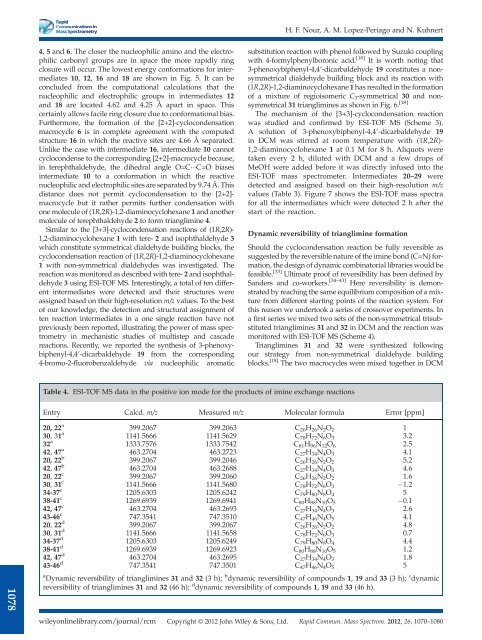
Table 4. ESI-TOF MS data in the positive ion mode for the products <strong>of</strong> imine exchange reactions<br />
Entry Calcd. m/z Measured m/z Molecular formula Error [ppm]<br />
1078<br />
20, 22 a 399.2067 399.2063 C 26 H 26 N 2 O 2 1<br />
30, 31 a 1141.5666 1141.5629 C 78 H 72 N 6 O 3 3.2<br />
32 a 1333.7576 1333.7542 C 81 H 96 N 12 O 6 2.5<br />
42, 47 a 463.2704 463.2723 C 27 H 34 N 4 O 3 4.1<br />
20, 22 b 399.2067 399.2046 C 26 H 26 N 2 O 2 5.2<br />
42, 47 b 463.2704 463.2688 C 27 H 34 N 4 O 3 4.6<br />
20, 22 c 399.2067 399.2060 C 26 H 26 N 2 O 2 1.6<br />
30, 31 c 1141.5666 1141.5680 C 78 H 72 N 6 O 3 1.2<br />
34-37 c 1205.6303 1205.6242 C 79 H 80 N 8 O 4 5<br />
38-41 c 1269.6939 1269.6941 C 80 H 88 N 10 O 5 0.1<br />
42, 47 c 463.2704 463.2693 C 27 H 34 N 4 O 3 2.6<br />
43-46 c 747.3541 747.3510 C 47 H 46 N 4 O 5 4.1<br />
20, 22 d 399.2067 399.2067 C 26 H 26 N 2 O 2 4.8<br />
30, 31 d 1141.5666 1141.5658 C 78 H 72 N 6 O 3 0.7<br />
34-37 d 1205.6303 1205.6249 C 79 H 80 N 8 O 4 4.4<br />
38-41 d 1269.6939 1269.6923 C 80 H 88 N 10 O 5 1.2<br />
42, 47 d 463.2704 463.2695 C 27 H 34 N 4 O 3 1.8<br />
43-46 d 747.3541 747.3501 C 47 H 46 N 4 O 5 5<br />
a Dynamic reversibility <strong>of</strong> trianglimines 31 and 32 (3 h); b dynamic reversibility <strong>of</strong> compounds 1, 19 and 33 (3 h); c dynamic<br />
reversibility <strong>of</strong> trianglimines 31 and 32 (46 h); d dynamic reversibility <strong>of</strong> compounds 1, 19 and 33 (46 h).<br />
wileyonlinelibrary.com/journal/rcm Copyright © 2012 John Wiley & Sons, Ltd. Rapid Commun. Mass Spectrom. 2012, 26, 1070–1080