The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
The Development of Novel Antibiotics Using ... - Jacobs University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
H. F. Nour, A. M. Lopez-Periago and N. Kuhnert<br />
Table 1. ESI-TOF MS data in the positive ion mode for the intermediates using terephthaldehyde 2, forming trianglimine 4<br />
Entry Calcd. m/z Measured m/z Molecular formula<br />
Error<br />
[ppm]<br />
4 637.4013 637.4012 C 42 H 48 N 6 0.2<br />
7 231.1492 231.1491 C 14 H 18 N 2 O 0.2<br />
8 327.2543 327.2544 C 20 H 30 N 4 0.3<br />
9 347.1754 347.1746 C 22 H 22 N 2 O 2 2.3<br />
10 443.2805 443.2813 C 28 H 34 N 4 O 1.7<br />
11 539.3857 539.3867 C 34 H 46 N 6 1.8<br />
12 655.4119 655.4115 C 42 H 50 N 6 O 0.6<br />
Table 2. ESI-TOF mass spectrometry data in the positive ion mode for the intermediates using isophthaldehyde 3, forming<br />
trianglimine 5<br />
Entry Calcd. m/z Measured m/z Molecular formula<br />
Error<br />
[ppm]<br />
6 425.2700 425.2715 C 28 H 32 N 4 3.7<br />
13 231.1492 231.1489 C 14 H 18 N 2 O 1.3<br />
14 327.2543 327.2537 C 20 H 30 N 4 2<br />
15 347.1745 347.1745 C 22 H 22 N 2 O 2 2.7<br />
16 443.2805 443.2807 C 28 H 34 N 4 O 0.4<br />
17 539.3857 539.3846 C 34 H 46 N 6 2<br />
18* 655.4119 655.4114 C 42 H 50 N 6 O 0.7<br />
*Intermediate was detected after 30 min from the start <strong>of</strong> the reaction.<br />
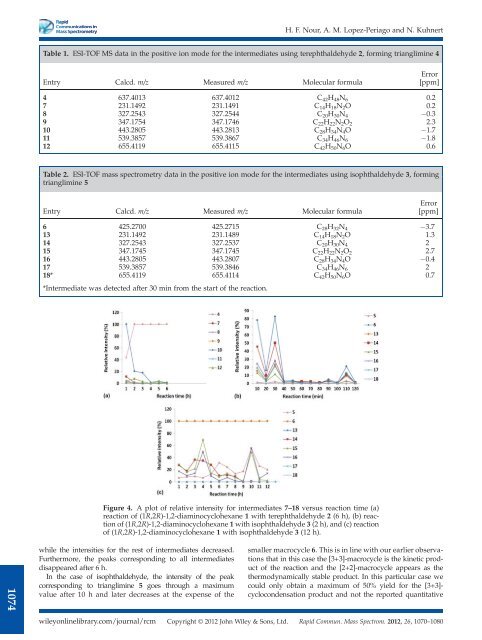
Figure 4. A plot <strong>of</strong> relative intensity for intermediates 7–18 versus reaction time (a)<br />
reaction <strong>of</strong> (1R,2R)-1,2-diaminocyclohexane 1 with terephthaldehyde 2 (6 h), (b) reaction<br />
<strong>of</strong> (1R,2R)-1,2-diaminocyclohexane 1 with isophthaldehyde 3 (2 h), and (c) reaction<br />
<strong>of</strong> (1R,2R)-1,2-diaminocyclohexane 1 with isophthaldehyde 3 (12 h).<br />
1074<br />
while the intensities for the rest <strong>of</strong> intermediates decreased.<br />
Furthermore, the peaks corresponding to all intermediates<br />
disappeared after 6 h.<br />
In the case <strong>of</strong> isophthaldehyde, the intensity <strong>of</strong> the peak<br />
corresponding to trianglimine 5 goes through a maximum<br />
value after 10 h and later decreases at the expense <strong>of</strong> the<br />
smaller macrocycle 6. This is in line with our earlier observations<br />
that in this case the [3+3]-macrocycle is the kinetic product<br />
<strong>of</strong> the reaction and the [2+2]-macrocycle appears as the<br />
thermodynamically stable product. In this particular case we<br />
could only obtain a maximum <strong>of</strong> 50% yield for the [3+3]-<br />
cyclocondensation product and not the reported quantitative<br />
wileyonlinelibrary.com/journal/rcm Copyright © 2012 John Wiley & Sons, Ltd. Rapid Commun. Mass Spectrom. 2012, 26, 1070–1080