2007_6_Nr6_EEMJ
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Syntheis, characterization and catalytic reduction of NOx emissions over LaMnO 3 perovskite<br />
60<br />
(c)<br />
50<br />
(b)<br />
(a)<br />
0 200 400 600 800 1000<br />
Conversia NO x<br />
[%]<br />
40<br />
30<br />
20<br />
10<br />
600 °C<br />
800 °C<br />
1000 °C<br />
Temperature (°C)<br />
0<br />
150 200 250 300 350 400 450 500 550 600 650<br />
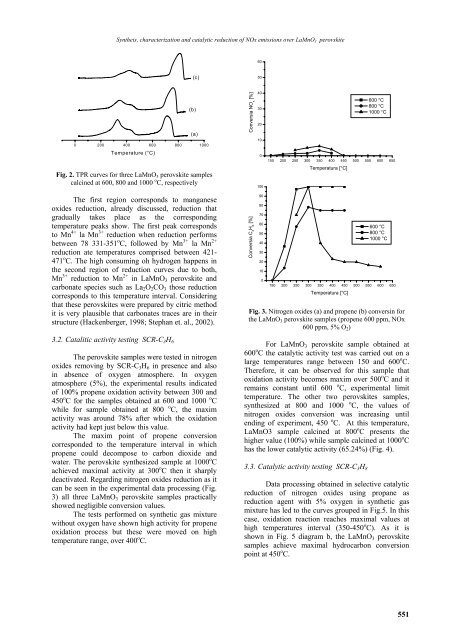
Fig. 2. TPR curves for three LaMnO 3 perovskite samples<br />
calcined at 600, 800 and 1000 o C, respectively<br />
100<br />
Temperatura [°C]<br />
The first region corresponds to manganese<br />
oxides reduction, already discussed, reduction that<br />
gradually takes place as the corresponding<br />
temperature peaks show. The first peak corresponds<br />
to Mn 4+ la Mn 3+ reduction when reduction performs<br />
between 78 331-351 o C, followed by Mn 3+ la Mn 2+<br />
reduction ate temperatures comprised between 421-<br />
471 o C. The high consuming oh hydrogen happens in<br />
the second region of reduction curves due to both,<br />
Mn 3+ reduction to Mn 2+ in LaMnO 3 perovskite and<br />
carbonate species such as La 2 O 2 CO 3 those reduction<br />
corresponds to this temperature interval. Considering<br />
that these perovskites were prepared by citric method<br />
it is very plausible that carbonates traces are in their<br />
structure (Hackenberger, 1998; Stephan et. al., 2002).<br />
3.2. Catalitic activity testing SCR-C 3 H 6<br />
The perovskite samples were tested in nitrogen<br />
oxides removing by SCR-C 3 H 6 in presence and also<br />
in absence of oxygen atmosphere. In oxygen<br />
atmosphere (5%), the experimental results indicated<br />
of 100% propene oxidation activity between 300 and<br />
450 o C for the samples obtained at 600 and 1000 o C<br />
while for sample obtained at 800 o C, the maxim<br />
activity was around 78% after which the oxidation<br />
activity had kept just below this value.<br />
The maxim point of propene conversion<br />
corresponded to the temperature interval in which<br />
propene could decompose to carbon dioxide and<br />
water. The perovskite synthesized sample at 1000 o C<br />
achieved maximal activity at 300 o C then it sharply<br />
deactivated. Regarding nitrogen oxides reduction as it<br />
can be seen in the experimental data processing (Fig.<br />
3) all three LaMnO 3 perovskite samples practically<br />
showed negligible conversion values.<br />
The tests performed on synthetic gas mixture<br />
without oxygen have shown high activity for propene<br />
oxidation process but these were moved on high<br />
temperature range, over 400 o C.<br />
Conversia C 3<br />
H 6<br />
[%]<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
600 °C<br />
800 °C<br />
1000 °C<br />
150 200 250 300 350 400 450 500 550 600 650<br />
Temperatura [°C]<br />
Fig. 3. Nitrogen oxides (a) and propene (b) conversin for<br />
the LaMnO 3 perovskite samples (propene 600 ppm, NOx<br />
600 ppm, 5% O 2 )<br />
For LaMnO 3 perovskite sample obtained at<br />
600 o C the catalytic activity test was carried out on a<br />
large temperatures range between 150 and 600 o C.<br />
Therefore, it can be observed for this sample that<br />
oxidation activity becomes maxim over 500 o C and it<br />
remains constant until 600 o C, experimental limit<br />
temperature. The other two perovskites samples,<br />
synthesized at 800 and 1000 o C, the values of<br />
nitrogen oxides conversion was increasing until<br />
ending of experiment, 450 o C. At this temperature,<br />
LaMnO3 sample calcined at 800 o C presents the<br />
higher value (100%) while sample calcined at 1000 o C<br />
has the lower catalytic activity (65.24%) (Fig. 4).<br />
3.3. Catalytic activity testing SCR-C 3 H 8<br />
Data processing obtained in selective catalytic<br />
reduction of nitrogen oxides using propane as<br />
reduction agent with 5% oxygen in synthetic gas<br />
mixture has led to the curves grouped in Fig.5. In this<br />
case, oxidation reaction reaches maximal values at<br />
high temperatures interval (350-450 o C). As it is<br />
shown in Fig. 5 diagram b, the LaMnO 3 perovskite<br />
samples achieve maximal hydrocarbon conversion<br />
point at 450 o C.<br />
551