Em4885 irrigation management practices to protect ground water
Em4885 irrigation management practices to protect ground water
Em4885 irrigation management practices to protect ground water
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3 CHAPTER<br />
Nitrogen as a Potential Pollutant<br />
Nitrogen is the most critical of the essential elements for plant growth. The addition of nitrogen<br />
fertilizer <strong>to</strong> enhance crop growth is basic <strong>to</strong> modern irrigated agriculture. The ultimate source of this<br />
nitrogen is the inert gas N which makes up about 78% of the atmosphere. However, except for<br />
2<br />
legumes, plants cannot use nitrogen in this form. Most plants use nitrogen in either the ammonium<br />
+ - - (NH ) form or the nitrate (NO3 ) form. Both occur in solution. However, the nitrate (NO3 ) form is<br />
4<br />
+ much more leachable than the ammonium (NH ) form.<br />
4<br />
Nitrogen is added <strong>to</strong> the soil in a number of ways:<br />
- 1. Lightning may cause the formation of the nitrate (NO ) form which falls <strong>to</strong> earth<br />
3<br />
with rainfall.<br />
2. Legumes such as alfalfa, soybeans, and clover can convert atmospheric N 2 in<strong>to</strong> usable<br />
form through “rhizobia” bacteria. Rhizobia exist in a symbiotic (mutually beneficial)<br />
relationship with the crop. They form and inhabit nodules (abnormal growths) on the<br />
root systems of legumes. This process is termed “symbiotic fixation.”<br />
3. “Non-symbiotic fixation” occurs through some types of blue-green algae and what are<br />
known as “free-living” bacteria. These require no other plants for existence. The amount<br />
of nitrogen fixed by non-symbiotic fixation is relatively small, with estimates in the range<br />
of ten pounds/acre annually.<br />
4. By far, the most important sources of plant-available nitrogen are through addition of<br />
commercial fertilizer and manure.<br />
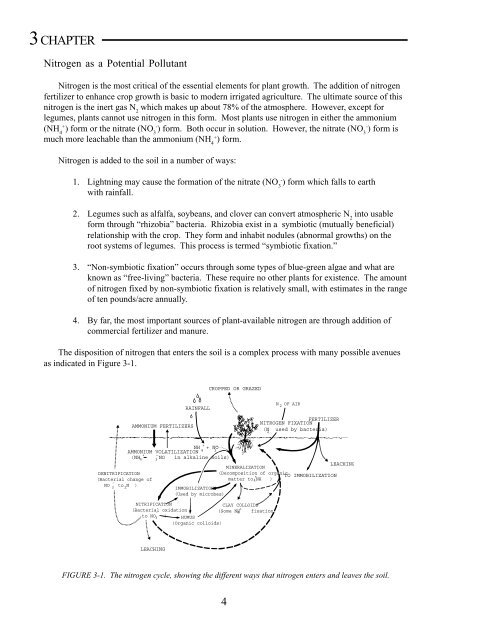
The disposition of nitrogen that enters the soil is a complex process with many possible avenues<br />
as indicated in Figure 3-1.<br />
AMMONIUM FERTILIZERS<br />
RAINFALL<br />
CROPPED OR GRAZED<br />
+ _<br />
NH + NO<br />
4 AMMONIUM VOLATILIZATION 3<br />
+ _<br />
(NH 4 3 NO in alkaline soils)<br />
MINERALIZATION<br />
LEACHING<br />
(Decomposition of organic<br />
TO IMMOBILIZATION<br />
matter <strong>to</strong> 4 NH )<br />
+<br />
DENITRIFICATION<br />
(Bacterial change of<br />
-<br />
NO 3 <strong>to</strong> 2<br />
N )<br />
IMMOBILIZATION<br />
(Used by microbes)<br />
NITRIFICATION<br />
(Bacterial oxidation<br />
<strong>to</strong> NO ) 3<br />
CLAY COLLOIDS<br />
-<br />
(Some NH 4 fixation)<br />
+<br />
HUMUS<br />
(Organic colloids)<br />
LEACHING<br />
FIGURE 3-1. The nitrogen cycle, showing the different ways that nitrogen enters and leaves the soil.<br />
4<br />
N OF AIR<br />
2<br />
FERTILIZER<br />
NITROGEN FIXATION<br />
(N used by bacteria)<br />
2