A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
150<br />
Appendix 4<br />
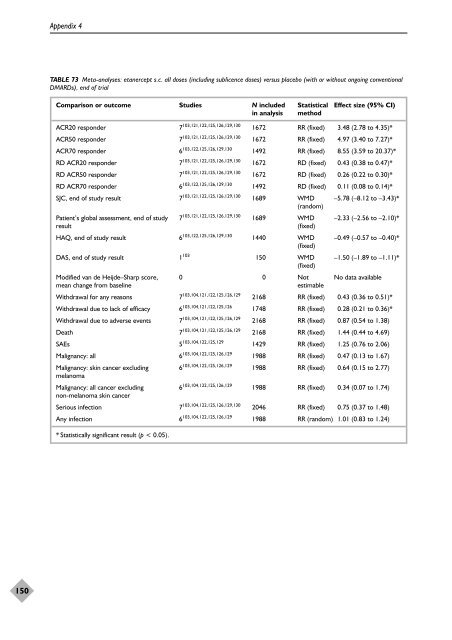
TABLE 73 Meta-analyses: etanercept s.c. all doses (including sublicence doses) versus placebo (with or without ongoing conventional<br />
DMARDs), end <strong>of</strong> trial<br />
Comparison or outcome Studies N included Statistical Effect size (95% CI)<br />
in analysis method<br />
ACR20 responder 7103,121,122,125,126,129,130 1672 RR (fixed) 3.48 (2.78 to 4.35)*<br />
ACR50 responder 7103,121,122,125,126,129,130 1672 RR (fixed) 4.97 (3.40 to 7.27)*<br />
ACR70 responder 6103,122,125,126,129,130 1492 RR (fixed) 8.55 (3.59 to 20.37)*<br />
RD ACR20 responder 7103,121,122,125,126,129,130 1672 RD (fixed) 0.43 (0.38 to 0.47)*<br />
RD ACR50 responder 7103,121,122,125,126,129,130 1672 RD (fixed) 0.26 (0.22 to 0.30)*<br />
RD ACR70 responder 6103,122,125,126,129,130 1492 RD (fixed) 0.11 (0.08 to 0.14)*<br />
SJC, end <strong>of</strong> study result 7103,121,122,125,126,129,130 1689 WMD<br />
(random)<br />
–5.78 (–8.12 to –3.43)*<br />
Patient’s global assessment, end <strong>of</strong> study 7 103,121,122,125,126,129,130 1689 WMD –2.33 (–2.56 to –2.10)*<br />
result (fixed)<br />
HAQ, end <strong>of</strong> study result 6 103,122,125,126,129,130 1440 WMD<br />
(fixed)<br />
–0.49 (–0.57 to –0.40)*<br />
DAS, end <strong>of</strong> study result 1 103 150 WMD<br />
(fixed)<br />
–1.50 (–1.89 to –1.11)*<br />
Modified van de Heijde–Sharp score, 0 0 Not No data available<br />
mean change from baseline estimable<br />
Withdrawal for any reasons 7 103,104,121,122,125,126,129 2168 RR (fixed) 0.43 (0.36 to 0.51)*<br />
Withdrawal due to lack <strong>of</strong> efficacy 6103,104,121,122,125,126 1748 RR (fixed) 0.28 (0.21 to 0.36)*<br />
Withdrawal due to adverse events 7103,104,121,122,125,126,129 2168 RR (fixed) 0.87 (0.54 to 1.38)<br />
Death 7103,104,121,122,125,126,129 2168 RR (fixed) 1.44 (0.44 to 4.69)<br />
SAEs 5103,104,122,125,129 1429 RR (fixed) 1.25 (0.76 to 2.06)<br />
Malignancy: all 6103,104,122,125,126,129 1988 RR (fixed) 0.47 (0.13 to 1.67)<br />
Malignancy: skin cancer excluding 6103,104,122,125,126,129 melanoma<br />
1988 RR (fixed) 0.64 (0.15 to 2.77)<br />
Malignancy: all cancer excluding 6103,104,122,125,126,129 non-melanoma skin cancer<br />
1988 RR (fixed) 0.34 (0.07 to 1.74)<br />
Serious infection 7103,104,122,125,126,129,130 2046 RR (fixed) 0.75 (0.37 to 1.48)<br />
Any infection 6103,104,122,125,126,129 1988 RR (random) 1.01 (0.83 to 1.24)<br />
* Statistically significant result (p < 0.05).