A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
54<br />
Effectiveness<br />
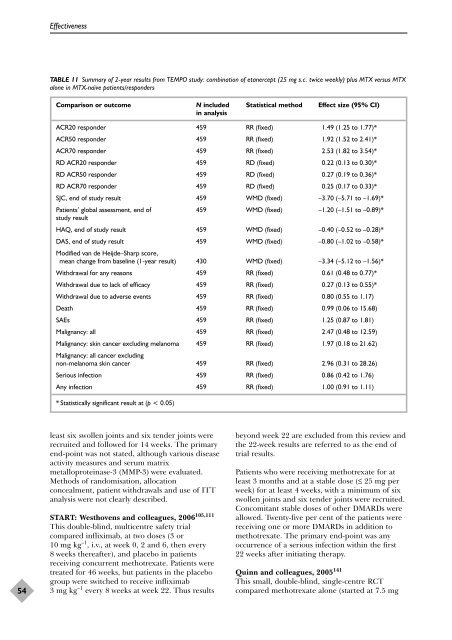
TABLE 11 Summary <strong>of</strong> 2-year results from TEMPO study: combination <strong>of</strong> etanercept (25 mg s.c. twice weekly) plus MTX versus MTX<br />
alone in MTX-naïve patients/responders<br />
Comparison or outcome N included Statistical method Effect size (95% CI)<br />
in analysis<br />
ACR20 responder 459 RR (fixed) 1.49 (1.25 to 1.77)*<br />
ACR50 responder 459 RR (fixed) 1.92 (1.52 to 2.41)*<br />
ACR70 responder 459 RR (fixed) 2.53 (1.82 to 3.54)*<br />
RD ACR20 responder 459 RD (fixed) 0.22 (0.13 to 0.30)*<br />
RD ACR50 responder 459 RD (fixed) 0.27 (0.19 to 0.36)*<br />
RD ACR70 responder 459 RD (fixed) 0.25 (0.17 to 0.33)*<br />
SJC, end <strong>of</strong> study result 459 WMD (fixed) –3.70 (–5.71 to –1.69)*<br />
Patients’ global assessment, end <strong>of</strong><br />
study result<br />
459 WMD (fixed) –1.20 (–1.51 to –0.89)*<br />
HAQ, end <strong>of</strong> study result 459 WMD (fixed) –0.40 (–0.52 to –0.28)*<br />
DAS, end <strong>of</strong> study result<br />
Modified van de Heijde–Sharp score,<br />
459 WMD (fixed) –0.80 (–1.02 to –0.58)*<br />
mean change from baseline (1-year result) 430 WMD (fixed) –3.34 (–5.12 to –1.56)*<br />
Withdrawal for any reasons 459 RR (fixed) 0.61 (0.48 to 0.77)*<br />
Withdrawal due to lack <strong>of</strong> efficacy 459 RR (fixed) 0.27 (0.13 to 0.55)*<br />
Withdrawal due to adverse events 459 RR (fixed) 0.80 (0.55 to 1.17)<br />
Death 459 RR (fixed) 0.99 (0.06 to 15.68)<br />
SAEs 459 RR (fixed) 1.25 (0.87 to 1.81)<br />
Malignancy: all 459 RR (fixed) 2.47 (0.48 to 12.59)<br />
Malignancy: skin cancer excluding melanoma<br />
Malignancy: all cancer excluding<br />
459 RR (fixed) 1.97 (0.18 to 21.62)<br />
non-melanoma skin cancer 459 RR (fixed) 2.96 (0.31 to 28.26)<br />
Serious infection 459 RR (fixed) 0.86 (0.42 to 1.76)<br />
Any infection 459 RR (fixed) 1.00 (0.91 to 1.11)<br />
* Statistically significant result at (p < 0.05)<br />
least six swollen joints and six tender joints were<br />
recruited and followed for 14 weeks. The primary<br />
end-point was not stated, although various disease<br />
activity measures and serum matrix<br />
metalloproteinase-3 (MMP-3) were evaluated.<br />
Methods <strong>of</strong> randomisation, allocation<br />
concealment, patient withdrawals and use <strong>of</strong> ITT<br />
analysis were not clearly described.<br />
START: Westhovens and colleagues, 2006 105,111<br />
This double-blind, multicentre safety trial<br />
compared infliximab, at two doses (3 or<br />
10 mg kg –1 , i.v., at week 0, 2 and 6, <strong>the</strong>n every<br />
8 weeks <strong>the</strong>reafter), and placebo in patients<br />
receiving concurrent methotrexate. Patients were<br />
treated for 46 weeks, but patients in <strong>the</strong> placebo<br />
group were switched to receive infliximab<br />
3mgkg –1 every 8 weeks at week 22. Thus results<br />
beyond week 22 are excluded from this <strong>review</strong> and<br />
<strong>the</strong> 22-week results are referred to as <strong>the</strong> end <strong>of</strong><br />
trial results.<br />
Patients who were receiving methotrexate for at<br />
least 3 months and at a stable dose (≤ 25 mg per<br />
week) for at least 4 weeks, with a minimum <strong>of</strong> six<br />
swollen joints and six tender joints were recruited.<br />
Concomitant stable doses <strong>of</strong> o<strong>the</strong>r DMARDs were<br />
allowed. Twenty-five per cent <strong>of</strong> <strong>the</strong> patients were<br />
receiving one or more DMARDs in addition to<br />
methotrexate. The primary end-point was any<br />
occurrence <strong>of</strong> a serious infection within <strong>the</strong> first<br />
22 weeks after initiating <strong>the</strong>rapy.<br />
Quinn and colleagues, 2005 141<br />
This small, double-blind, single-centre RCT<br />
compared methotrexate alone (started at 7.5 mg