A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
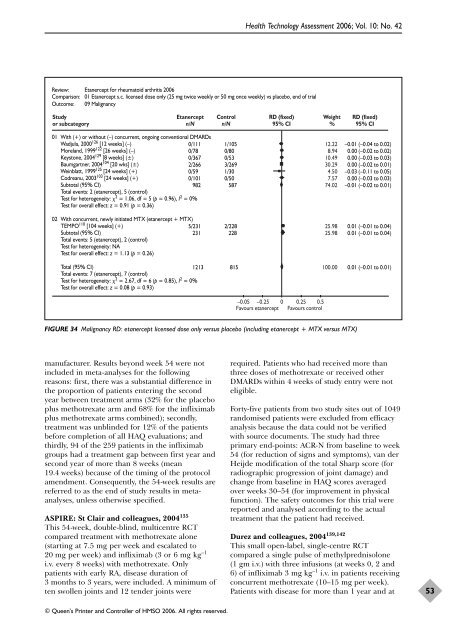
Review: Etanercept for rheumatoid arthritis 2006<br />
Comparison: 01 Etanercept s.c. licensed dose only (25 mg twice weekly or 50 mg once weekly) vs placebo, end <strong>of</strong> trial<br />
Outcome: 09 Malignancy<br />
Study<br />
or subcategory<br />
Etanercept<br />
n/N<br />
manufacturer. Results beyond week 54 were not<br />
included in meta-analyses for <strong>the</strong> following<br />
reasons: first, <strong>the</strong>re was a substantial difference in<br />
<strong>the</strong> proportion <strong>of</strong> patients entering <strong>the</strong> second<br />
year between treatment arms (32% for <strong>the</strong> placebo<br />
plus methotrexate arm and 68% for <strong>the</strong> infliximab<br />
plus methotrexate arms combined); secondly,<br />
treatment was unblinded for 12% <strong>of</strong> <strong>the</strong> patients<br />
before completion <strong>of</strong> all HAQ evaluations; and<br />
thirdly, 94 <strong>of</strong> <strong>the</strong> 259 patients in <strong>the</strong> infliximab<br />
groups had a treatment gap between first year and<br />
second year <strong>of</strong> more than 8 weeks (mean<br />
19.4 weeks) because <strong>of</strong> <strong>the</strong> timing <strong>of</strong> <strong>the</strong> protocol<br />
amendment. Consequently, <strong>the</strong> 54-week results are<br />
referred to as <strong>the</strong> end <strong>of</strong> study results in metaanalyses,<br />
unless o<strong>the</strong>rwise specified.<br />
ASPIRE: St Clair and colleagues, 2004 135<br />
This 54-week, double-blind, multicentre RCT<br />
compared treatment with methotrexate alone<br />
(starting at 7.5 mg per week and escalated to<br />
20 mg per week) and infliximab (3 or 6 mg kg –1<br />
i.v. every 8 weeks) with methotrexate. Only<br />
patients with early RA, disease duration <strong>of</strong><br />
3 months to 3 years, were included. A minimum <strong>of</strong><br />
ten swollen joints and 12 tender joints were<br />
Control<br />
n/N<br />
01 With (+) or without (–) concurrent, ongoing conventional DMARDs<br />
Wadjula, 2000126 [12 weeks] (–)<br />
Moreland, 1999122 [26 weeks] (–)<br />
Keystone, 2004129 [8 weeks] (±)<br />
Baumgartner, 2004104 [20 wks] (±)<br />
Weinblatt, 1999125 [24 weeks] (+)<br />
Codreanu, 2003103 [24 weeks] (+)<br />
Subtotal (95% CI)<br />
Total events: 2 (etanercept), 5 (control)<br />
Test for heterogeneity: 2 = 1.06, df = 5 (p = 0.96), I2 0/111<br />
0/78<br />
0/367<br />
2/266<br />
0/59<br />
0/101<br />
1/105<br />
0/80<br />
0/53<br />
3/269<br />
1/30<br />
0/50<br />
982 587<br />
= 0%<br />
Test for overall effect: z = 0.91 (p = 0.36)<br />
02 With concurrent, newly initiated MTX (etanercept + MTX)<br />
TEMPO 110 [104 weeks] (+)<br />
Subtotal (95% CI)<br />
Total events: 5 (etanercept), 2 (control)<br />
Test for heterogeneity: NA<br />
Test for overall effect: z = 1.13 (p = 0.26)<br />
5/231 2/228<br />
231 228<br />
Total (95% CI)<br />
Total events: 7 (etanercept), 7 (control)<br />
Test for heterogeneity: 2 = 2.67, df = 6 (p = 0.85), I2 1213 815<br />
= 0%<br />
Test for overall effect: z = 0.08 (p = 0.93)<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
RD (fixed)<br />
95% CI<br />
–0.05 –0.25 0 0.25 0.5<br />
Favours etanercept Favours control<br />
Weight<br />
%<br />
FIGURE 34 Malignancy RD: etanercept licensed dose only versus placebo (including etanercept + MTX versus MTX)<br />
12.22<br />
8.94<br />
10.49<br />
30.29<br />
4.50<br />
7.57<br />
74.02<br />
25.98<br />
25.98<br />
100.00<br />
RD (fixed)<br />
95% CI<br />
–0.01 (–0.04 to 0.02)<br />
0.00 (–0.02 to 0.02)<br />
0.00 (–0.03 to 0.03)<br />
0.00 (–0.02 to 0.01)<br />
–0.03 (–0.11 to 0.05)<br />
0.00 (–0.03 to 0.03)<br />
–0.01 (–0.02 to 0.01)<br />
0.01 (–0.01 to 0.04)<br />
0.01 (–0.01 to 0.04)<br />
0.01 (–0.01 to 0.01)<br />
required. Patients who had received more than<br />
three doses <strong>of</strong> methotrexate or received o<strong>the</strong>r<br />
DMARDs within 4 weeks <strong>of</strong> study entry were not<br />
eligible.<br />
Forty-five patients from two study sites out <strong>of</strong> 1049<br />
randomised patients were excluded from efficacy<br />
analysis because <strong>the</strong> data could not be verified<br />
with source documents. The study had three<br />
primary end-points: ACR-N from baseline to week<br />
54 (for reduction <strong>of</strong> signs and symptoms), van der<br />
Heijde modification <strong>of</strong> <strong>the</strong> total Sharp score (for<br />
radiographic progression <strong>of</strong> joint damage) and<br />
change from baseline in HAQ scores averaged<br />
over weeks 30–54 (for improvement in physical<br />
function). The safety outcomes for this trial were<br />
reported and analysed according to <strong>the</strong> actual<br />
treatment that <strong>the</strong> patient had received.<br />
Durez and colleagues, 2004 139,142<br />
This small open-label, single-centre RCT<br />
compared a single pulse <strong>of</strong> methylprednisolone<br />
(1 gm i.v.) with three infusions (at weeks 0, 2 and<br />
6) <strong>of</strong> infliximab 3 mg kg –1 i.v. in patients receiving<br />
concurrent methotrexate (10–15 mg per week).<br />
Patients with disease for more than 1 year and at<br />
53