A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
40<br />
Effectiveness<br />
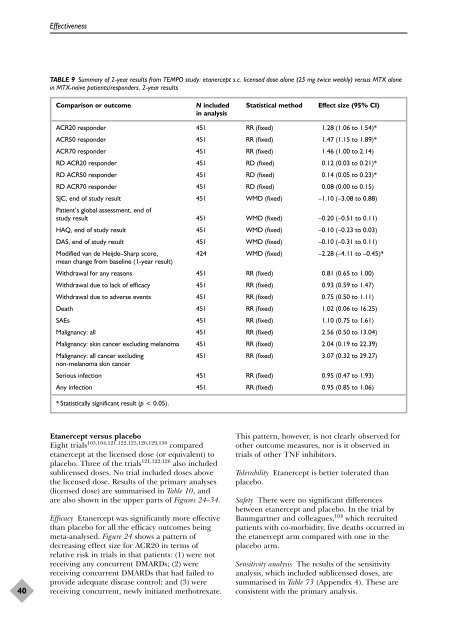
TABLE 9 Summary <strong>of</strong> 2-year results from TEMPO study: etanercept s.c. licensed dose alone (25 mg twice weekly) versus MTX alone<br />
in MTX-naïve patients/responders, 2-year results<br />
Comparison or outcome N included Statistical method Effect size (95% CI)<br />
in analysis<br />
ACR20 responder 451 RR (fixed) 1.28 (1.06 to 1.54)*<br />
ACR50 responder 451 RR (fixed) 1.47 (1.15 to 1.89)*<br />
ACR70 responder 451 RR (fixed) 1.46 (1.00 to 2.14)<br />
RD ACR20 responder 451 RD (fixed) 0.12 (0.03 to 0.21)*<br />
RD ACR50 responder 451 RD (fixed) 0.14 (0.05 to 0.23)*<br />
RD ACR70 responder 451 RD (fixed) 0.08 (0.00 to 0.15)<br />
SJC, end <strong>of</strong> study result<br />
Patient’s global assessment, end <strong>of</strong><br />
451 WMD (fixed) –1.10 (–3.08 to 0.88)<br />
study result 451 WMD (fixed) –0.20 (–0.51 to 0.11)<br />
HAQ, end <strong>of</strong> study result 451 WMD (fixed) –0.10 (–0.23 to 0.03)<br />
DAS, end <strong>of</strong> study result 451 WMD (fixed) –0.10 (–0.31 to 0.11)<br />
Modified van de Heijde–Sharp score,<br />
mean change from baseline (1-year result)<br />
424 WMD (fixed) –2.28 (–4.11 to –0.45)*<br />
Withdrawal for any reasons 451 RR (fixed) 0.81 (0.65 to 1.00)<br />
Withdrawal due to lack <strong>of</strong> efficacy 451 RR (fixed) 0.93 (0.59 to 1.47)<br />
Withdrawal due to adverse events 451 RR (fixed) 0.75 (0.50 to 1.11)<br />
Death 451 RR (fixed) 1.02 (0.06 to 16.25)<br />
SAEs 451 RR (fixed) 1.10 (0.75 to 1.61)<br />
Malignancy: all 451 RR (fixed) 2.56 (0.50 to 13.04)<br />
Malignancy: skin cancer excluding melanoma 451 RR (fixed) 2.04 (0.19 to 22.39)<br />
Malignancy: all cancer excluding<br />
non-melanoma skin cancer<br />
451 RR (fixed) 3.07 (0.32 to 29.27)<br />
Serious infection 451 RR (fixed) 0.95 (0.47 to 1.93)<br />
Any infection 451 RR (fixed) 0.95 (0.85 to 1.06)<br />
* Statistically significant result (p < 0.05).<br />
Etanercept versus placebo<br />
Eight trials 103,104,121,122,125,126,129,130 compared<br />
etanercept at <strong>the</strong> licensed dose (or equivalent) to<br />
placebo. Three <strong>of</strong> <strong>the</strong> trials 121,122,126 also included<br />
sublicensed doses. No trial included doses above<br />
<strong>the</strong> licensed dose. Results <strong>of</strong> <strong>the</strong> primary analyses<br />
(licensed dose) are summarised in Table 10, and<br />
are also shown in <strong>the</strong> upper parts <strong>of</strong> Figures 24–34.<br />
Efficacy Etanercept was significantly more effective<br />
than placebo for all <strong>the</strong> efficacy outcomes being<br />
meta-analysed. Figure 24 shows a pattern <strong>of</strong><br />
decreasing effect size for ACR20 in terms <strong>of</strong><br />
relative risk in trials in that patients: (1) were not<br />
receiving any concurrent DMARDs; (2) were<br />
receiving concurrent DMARDs that had failed to<br />
provide adequate disease control; and (3) were<br />
receiving concurrent, newly initiated methotrexate.<br />
This pattern, however, is not clearly observed for<br />
o<strong>the</strong>r outcome measures, nor is it observed in<br />
trials <strong>of</strong> o<strong>the</strong>r TNF inhibitors.<br />
Tolerability Etanercept is better tolerated than<br />
placebo.<br />
Safety There were no significant differences<br />
between etanercept and placebo. In <strong>the</strong> trial by<br />
Baumgartner and colleagues, 104 which recruited<br />
patients with co-morbidity, five deaths occurred in<br />
<strong>the</strong> etanercept arm compared with one in <strong>the</strong><br />
placebo arm.<br />
Sensitivity analysis The results <strong>of</strong> <strong>the</strong> sensitivity<br />
analysis, which included sublicensed doses, are<br />
summarised in Table 73 (Appendix 4). These are<br />
consistent with <strong>the</strong> primary analysis.